Review of Palaeobotany and Palynology, 7l (1992): 269-294 Elsevier Science Publishers B. V., Amsterdam
Angiosperm-like pollen from the ammonite-dated Oxfordian
(Upper Jurassic) of France
Bruce Corneta and Daniel Habibb
aLamont-Doherty Geological Observatory of Columbia University, Palisades, NY 10964, USA
bDepartment of Geology, Queens College, Flushing, NY 11367, USA
(Received August 17, 1990; revised and accepted November 25,1991)ABSTRACT
Cornet, B. and Habib, D., 1992. Angiosperm-like pollen from the ammonite-dated Oxfordian (Upper Jurassic) of France. Rev. Palaeobot. Palynol., 71: 269-294.
Two species of angiosperm-like pollen are described from an outcrop of lower Oxfordian Oxford Clay at Normandy, France. An Oxfordian age is established by ammonites (Quenstedtoceras mariae zone) and substantiated by associated age-diagnostic dinoflagellates. The angiosperm-like taxa are determined to be in place by their state of preservation and unique morphology. The more abundant taxon, Stellatopollis pocockii n. sp., closely resembles the pollen of extant Lilium bulbiferum in size and sculpture, but also closely resembles similar but smaller pollen found in the Lower Cretaceous. Stellatopollis Doyle 1975 is emended to include a wider range of Early Cretaceous reticulate monosulcate grains with crotonoid sculpture. A diporate species, Multimarginites sp. A, closely resembling the pollen of extant Bravaisia and Sanchezia (Acanthaceae) in size and sculpture is described and compared to a similar morphotype (Cornetipollis) from the late Triassic Richmond basin of Virginia. The genus Multimarginites Germeraad, Hopping et Muller 1968 is emended as di- or triporate and polyplicate, since its multimarginate "colpi" are non-apertural harmomegathic furrows. The significance of these unusual pollen types in pre-Barremian strata is discussed and they are placed in perspective with angiosperm-like pollen and megafossils from the Late Triassic of North America. The new combination Corollina chateaunovii (Reyre) novo com. was made.
Introduction
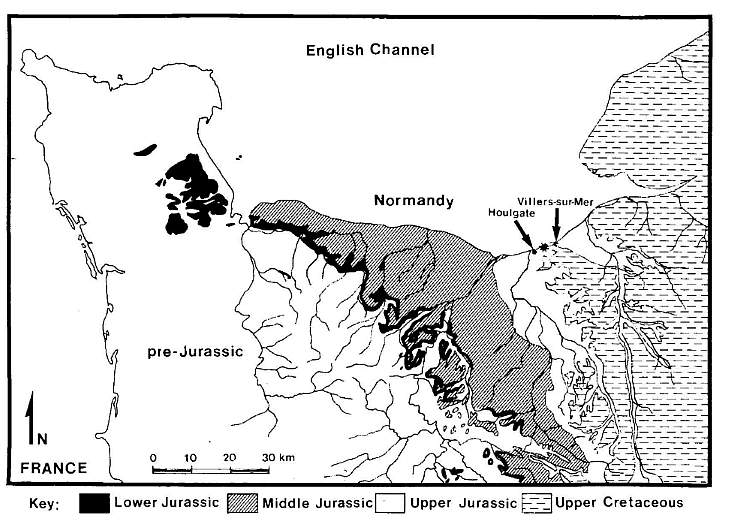
Outcrop samples from the type section area for the Oxfordian between Houlgate and Villers-sur-Mer in Normandy (France) were made available to the senior author in 1979 while working for Gulf Research & Development Company; Houston (Fig. 1). During the routine study of palynoflorules recovered from those samples, angiosperm-like pollen was discovered in one of them (Fig.2: sample 1053). The specimens (representing two taxa) were large enough (69-138 um). that they were easily spotted in strew preparations, and common enough that 36 were found. These specimens were all flattened by compression, as were all the other palynomorphs in the sample, and showed a range of preservation from very well preserved to substantially degraded. A significant percentage of the Stellatopollis specimens (twelve specimens in four clumps) were found joined, having survived sample preparation because their exines were fused where the pollen grains made contact with one another. The presence of impression marks of mineral grains on some of the specimens, along with their state of preservation and relative abundance (for pre-Barremian angiosperm-like pollen), indicate that they are in place and not contaminants.
Materials and methods
All specimens used in this study come from outcrop sample 1053, collected from exposures of Oxford Clay located between Houlgate and Villers-sur-Mer in Normandy, France (Figs.l,2). Sample 1053 (a calcareous gray mudstone) and 43 other rock samples of Oxford Clay (numbered consecutively from 1014 to 1067), were provided by Prof. Thomas Barnard to Gulf Research & Developmenr Co. (now Chevron Co. USA) as part of a collection for the micropaleontological and palynological study of type Jurassic sections in Europe. Most of the described specimens (including dinocysts) were prepared as single or multiple grain mounts and were picked from the two organic residues prepared from cuts of sample 1053. Strew slides of both residues were also made for palynoflorule study. No additional cuts were processed due to the small size of the remaining sample, which was kept for future backup study (USNM455251). All specimens were photographed with a Zeiss photomicroscope. Palynological residues were first prepared by a technician at the Gulf Research & Developmenty laboratory using standard chemical and mechanical (centrifuge) techniques of rock sample maceration in HCl, then in HF, with heavy liquid separation using zinc bromide, and 10 minutes oxidation in dilute bleach. Palynological residues and. picked grains were mounted on slides using glycerine jelly with a trace of phenol as preservative. Drops of a glycerol-water mounting solution containing picked grains were sealed with wax under 22 mm square cover slips on standard glass slides.

Fig. 1. Geologic map of Normandy, France, showing the approximate location of sample 1053 between Houlgate and Villers-sur-Mer, south shore of the English Channel. Adapted from 1955 edition of geologic map of France: scale 1 : 1,000,000, by Service de la Carte Géologique dela France.
All strew slides and single or multiple grain mounts are deposited at the National Museum of Natural History - Smithsonian Institution in Washington, D.C. under the care of James P. Ferrigno. Single and multiple grain mounts of Stellatopollis pocockii n. sp. were given USNM catalog numbers 455208-455213; 455216-455218;
455219-455224 (these last six specimens disinteg'rated on the slides as of 6-4-91). Single grain mounts of Multimarginites sp. A were given USNM catalog numbers 455213-455215 (specimen 455214 also disintegrated). USNM numbers are provided in corresponding photographic figure captions. Single and multiple grain mounts of spores, pollen and dinoflagellates were given the following USNM catalog numbers: 455200 (Sample 1030: dinoflagellate cysts); 455225-455231 (Sample 1053: spores and pollen); 455232-455250 (Sample 1053: dinoflagellate cysts). The remaining unprocessed.rock sample in a small manila envelope is given USNM catalog no. 455251. The remaining processed palynological residue, which was picked for angiosperm-like pollen, is stored in a water/alcohol/glycerine solution with 5% phenol in a small glass vial and is given USNM catalog no. 455252.
Fig.2. Stratigraphic section of the Oxford Clay between Houlgate and Villers-sur-Mer, showing the location of sample 1053. Based on data provided by T. Barnard to Gulf Oil Corp.
Fourteen strew slides prepared from 12 outcrop samples of Oxford. Clay are included in the slide collection deposited at the National Museum of Natural History, W.D.C., USA, and are listed below: Gulf Oil Corp. sample nos.1014 and 1019 (USNM455193 and USNM455194) from Quenstedtoceras lamberti ammonite zone (late Callovian); Gulf Oil Corp. sample nos.1023, 1027, 1029, 1030, 1032, 1038, 1042, 1045, 1048 and 1053 (USNM455195 through USNM455207) from Q. mariae ammonite zone (early Oxfordian). Slide with catalog no.USNM455207 contains a specimen of S. pocockii n. sp., which is indicated by a black dot on cover slip. A figure showing the stratigraphic section of Oxford Clay with locations of above numbered samples was provided with the side collection. Photographs of original sample were also provided with collection.
Specimens of Lilium bulbiferum pollen were used for comparison with S. pocockii n. sp.; they were derived from a greenhouse plant purchased in Houston, Texas, which was grown from bulb.
The possibility of contamination with recent pollen is remote, particularly since the types of pollen described here are so unusual. The first cut of outcrop sample 1053 was processed by a technician, while the second was done by the senior author, making it highly unlikely that angiosperm-like grains found in organic residues from both cuts were contaminants. Most other samples processed in the laboratory were well cuttings, which had drilling mud solids on their surfaces. Routinely, the first products of digestion in HCl were discarded by the technician to remove possible surface contaminants. In addition, the first products of digestion in HF were discarded by the senior author for the cut he processed to insure that surface contaminants were not present. Furthermore, there are slight but recognizable structural differences between these taxa and their modern look-a-likes.
Age of the sample
Sample 1053 comes from exposures of Oxford Clay in beach-side cliffs along the southern margin of the English Channel, France (Fig.2). The sample, according to data provided by T. Barnard, came from the upper third of the Quenstedtoceras mariae ammonite zone, which occupies the lower detailed systematic treatment and one new combi nation are indicated with an asterisk. Table IV gives the relative percentages of morphological groups or genera encountered in a count of 235 palynomorphs. The relative percentages of individual pollen and spore species for that count are given in Tables II and III. Based on that count, 84.7% are pollen, 9.4% are spores, 5.1% are dinoflagellates and 0.8% are acritarchs.
Fig.3. Stratigraphic datums of nine dinoflogellate species in the ammonite zonal sequence of the English Jurassic. FAD indicated by
; LAD by T. Late Callovian ammonite designation after Riley and Fenton, 1982.
TABLE IIAlphabetical listing of spore species found in palynoflorule 1053; [relative percentages] based on count of 235 palynomorphs.
Anapiculatisporites dawsonensis Reiser et Williams 1969
Antulsporites clavus (Balme 1957) Filatoff 1975
Concavissimisporites subgranulosus (Couper 1958) Pocock 1970 [0.8%]
Contignisporites cooksonii (Balme 1957) Dettmann 1963
Converrucosis porites variverrucatus Couper 1958 [0.8%]
Cyathidites minor Couper 1958 [0.8%]
Densoisporites perinatus Couper 1958 [0.4%]
Densoisporites velatus Weyland et Kreiger 1953
Densoisporites sp. A (Filatoff, 1975)
Dictyophyllidites harrisii Couper 1958 [1.7%]
Foraminisporis jurassica E. Schulz 1967 [0.4%]
Gleicheniidites senonicus Ross 1949
Granulatisporites injirmus (Balme 1957) Cornet et Traverse 1975 [0.4%]
Foraminisporis jurassica E. Schulz 1967 ..
Harrisispora equiexinus (Couper 1958) Pocock 1970
Heliosporites altmarkensis E. Schulz 1962 sensu Srivastava (1987)
Ischyosporites marburgensis De Jersey 1963
Klukisporiies lacunus Filatoff 1975
Klukisporites variegatus Couper 1958
cf. Kraeuselisporites hostilobatus Playford 1971
Leptolepidites major Couper 1958
Leptolepidites rotundus Tralau 1968
Lycopodiumsporites cf. L. austroclavatidites (Cookson 1953) Potonie 1956 [0.4%]
Lygodioisporites perverrucatus Couper 1958
Matonisporites crassiangulatus (Balme 1957) Dettmann 1963
Osmundacidites wellmannii Couper 1958 [0.8%]
Staplinisporites mathusii (Srivastava 1966) Filatoff 1975
Todisporites minor Couper 1958 [1.3%]
TABLE III
Alphabetical listing of pollen species found in palynoflorule 1053; [relative percentages] based on count of 235 palynomorphs.
Alisporites grandis (Cookson 1953) Dettmann 1963
Alisporites parvus De Jersey 1962 [2.1%]
Alisporites rotundus Rouse 1959
Alisporites thomasii (Couper 1958) Pocock 1962 [5.1 %]
Araucariacites australis Cookson 1947 [4.7%]
Araucariacites fissus Reiser et Williams 1969 [1.7%]
Araucariacites punctatus (Nilsson 1958) Cornet et Traverse 1975 [1.7%]
Balmeiopsis turbatus (Balme 1957) Srivastava 1987
Callialasporites dampieri (Balme 1957) Sukh Dev 1961
Callialasporites segmentatus (Balme 1957) Cornet et Traverse 1975 [0.4%]
Cerebropollenites macroverrucosus (Thiergart 1949) E. Schulz 1967 [0.4%]
Cerebropollenites theirgartii E. Schulz 1967 [1.7%]
Clavatipollenites sp. (minute connate sculpture on muri; many small columellae; lumina enlarged equatorially)
Corollina simplex (Danze-Corsin et Laveine 1963) Cornet et Traverse 1975
Corollina torosa (Reissinger 1950) Klaus 1960 [8.1%]
Corollina chateaunovii (Reyre 1970) comb. nov.* [0.4%] Basionym; Classopollis chateaunovii Reyre 1970
Cycadopites nitidus (Balme 1957) Norris 1969 [4.7%]
Cycadopites westfieldicus Cornet et Traverse 1975
Cycadopites sp. (Clavatipollenites hughesii sensu Vigran et Thusu, 1975: with well-developed alveolar
intrastructure) USNM455225 [0.4%]
Duplicisporites sp. A (Srivastava, 1987) [2.1%]
Eucommiidites sp. [1.7%]
Exesipollenites scabratus (Couper 1958) Pocock 1970 (8:5%]
Exesipollenites tumulus Balme 1957 [21.3%]
Multimarginites sp. A *
Perinopollenites elatoides Couper 1958 [11.9%]
Pinuspollenites parvisaccatus (De Jersey 1959) Filatoff 1975 [3.0%]
Podocarpidites ellipticus Cookson 1947 ex Couper 1958
Podocarpidites langii Pocock 1970
Spheripollenites psilatus Couper 1958
Stellatopollis pocockii n. sp.* [0.4%]
Vitreisporites pallidus Reissinger 1950 emend. Nilsson 1958 [0.4%]
The pollen and spore flora (Tables II and III) is essentially identical to that commonly described from the Callovian to Kimmeridgian of Europe and elsewhere (e.g. Couper, 1958; Herngreen and De Boer, 1974; Filatoff, 1975; Vigran and Thusu, 1975; Srivastava, 1987). The most abundant taxa are Araucariacites australis, A. fissus, Corollina torosa, Exesipollenites tumulus, E. scabratus and Perinopollenites elatoides. In some of the other palynoflorules from this outcrop of Oxford Clay, Callialasporites spp. and bisaccate pollen are not common. Some taxa present in palynoflorule 1053 are normally restricted to older Jurassic strata (e.g. Ischyosporites marburgensis and Heliosporites altmarkensis), indicating either reworking from a nearby source, possibly to the west (Fig.2), or an extension of their normal range. Most of.the 25 spore and 28 pollen taxa are long ranging, and are generally recognized as being of little value in establishing an age more definitite than Middle to Late Jurassic. The absence of any spore or pollen taxa diagnostic of the Cretaceous is consistent with an Oxfordian age based on dinoflagellates and ammonites.
TABLE IV
Relative percentages of morphological groups or genera, based on count of 235 palynomorphs (slide #USNM 455207)
araucarian pollen (e.g, Callialasporites)
medium to large bisaccate pollen (e.g. Alisporites)
small bisaccate pollen (< 40 um; e,g. Vitreisporites)
Cerebropollenites spp.
Perinopollenites spp.
circumpolles pollen (e.g. Corollina; Dup/icisporites)
Exesipollenites spp.
simple monosulcate pollen
Eucommiidites spp.
angiosperm-like pollen (e.g. Stellatopollis)
unidentified pollen
lycopod or cingulate spores (e.g. Densoisporites)
psilate spores (e.g. Dictyophyllidites)
other sculptured spores (e.g. Converrucosisporites)
unidentified spores
dinoflagellates
acritarchs8.5%
10.2%
0.4%
2.1%
11.9%
10.6%
29.8%
5.5%
1.7%
0.4%
3.6%
0.8%
5.0%
3.2%
0.4%
5.1%
0.8%
100.0%
Systematic palynology
All fossil pollen specimens either illustrated herein or used in the systematic part of this study are deposited in the palynological collections of the National Museum of Natural History in Washington, D.C., USA. The slides containing the holotype and paratype of Stellatopollis pocockii n. sp. have been appropriately labelled and designated in the text and plates.
Genus Stellatopollis Doyle 1975 emend.
Type species: Stellatopollis barghoornii Doyle 1975
.'
Emended diagnosis: Pollen grains monosulcate; exine tectate to semitectate, reticulate, with muri of reticulum-bearing crowded or uniformly spaced supratectal projections, whose heads are triangular to elliptical or subrectangular in surface view; columellae supporting reticulum narrow or wide, uniformly spaced or crowded.
Remarks: Doyle's original description considered only one species. The sculpture of S. barghoornii is similar to crotonoid sculptures of several pollen taxa from the Barremian (e.g. Hughes et al., 1979), but differs in organization. Either the generic diagnosis for Stellatopollis should be restricted to include only S. barghoornii, or it should be broadened so that the organization of supratectal sculpture can be used to distinguish species. Not all morphologically similar taxa are semitectate, nor do their supratectal sculptures consist dom:inantly of triangular elements as in S. barghoornii. In many taxa, triangular elements appear to be concentrated where the muri of adjacent brochi or lumina join, while oval to subrectangular elements tend to occupy spaces on the muri between these junctures. Supratectal elements can be crowded to the point of contact and even fusion, or they can be widely spaced as in the type species. Thus, the circumscription of the genus has been expanded here to include many of the monosulcate taxa with crotonoid sculptures found in the Lower Cretaceous, including those that superficially resemble the pollen of Lilium (cf. Liliipollis Krutzsch 1979). Penny (1986) described two new Stellatopollis species (S. hughesii and S. bituberensis) from the ?late Barremian of Egypt. The difference in sculpture between these two species exemplifies the variability circumscribed by the above emendation.Stellatopollis pocockii n. sp. - (Plate II, 1, 3-6; Plate III, 2; Plate IV, 1,2; Plate V, 1-4)
Holotype: Plate II, 1. Slide USNM455219. Dimensions 92 x 57 um.
Paratype: Slide USNM455218. Dimensions 72 x 52 um.
Diagnosis: Pollen grains monosulcate; corpus oblong to elliptical. Monosulcus rounded at ends, narrower at distal pole. Exine reticulate-columlllate, semitectate with collumellae attached to nexine (nexine and sexine stain the same with safranin 0). Lumina of reticulum coarsest proximally and laterally, with lumina decreasing in size toward grain apices and along sulcus margins. Proximal and lateral lumina subrounded to irregular in shape, frequently polygonal with straight sides; lumina range in length (longest dimension) from 1.0 um (average 4.9) to 12.0 um. Lumina at grain apices and along sulcus margin typically less than three um wide. Sculpture crotonoid with crowded (frequently touching) supratectal elements, which vary in surface view from triangular to subrectangular or elliptical. Triangular elements 1.0-2.2 um wide, usually positioned at junctions of muri, while subrectangular to elliptical elements 0.9-2.1 um long, usually positioned along muri common to only two adjacent lumina. Supratectal elements 0.74-0.99 um tall, bulge over and conceal muri - laterally muri only as wide as underlying columellae. Exine about 2.0-2.5 um thick; sexine tl.O- 2.0 um thick with columellae 0.44-0.49 um tall, 0.22-(average 0.54)-0.86 um wide; nexine about 0.5-0.8 um thick. Grana and verrucae occur on the floor of the lumina (i.e. attached to the nexine).
Dimensions (33 specimens): 69-(average 91.7)-105 um in length; 41-(average 55.8)-84 um in width.
Derivatio nominis: pocockii - after Dr. Stanley A.J. Pocock, whose name is synonymous with Jurassic palynology, for his significant contributions to our understanding of Jurassic palynofloras of North America.
Remarks: Two specimens (one in Plate IV, 1) showed possible endexine preserved under aperuture. Unlike the sexine, the endexine-like bodies stained very strongly with safranin 0; their unstained color was pale yellow. Columellae are clearly evident in the SEM micrographs (Plate V), although they are dificult to distinguish with transmitted light. The columellae in one specimen (Plate V, 1,2: arrows) are regularly spaced and appear to be arranged mostly one-on-one with the supratectal elements, while in another specimen (Plate V, 3,4: arrows) they are crowded, vary considerably in width (0.22-0.86 um), and are clearly not coordinated with the overlying supratectal elements. In Stellatopollis barghoornii Doyle (in Doyle et al., 1975) the columellae are much smaller and narrower than those of S. pocockii n. sp., and are not coordinated with the supratectal elements. In Recent Lilium bulbiferum pollen (Plate II, 2; Plate III, 1) supratectal elements are more widely spaced and columellae are short and narrow as in S. barghoornii. Subrectangular elements are also present as in S. pocockii n. sp. In all three taxa the supratectal elements ("clavate projections" of Doyle: in Doyle et al., 1975) are connected by ribbon-like "bridges" or narrow muri. S. pocockii n. sp. may be less derived than Cretaceous species of Stellatopollis, because there is a greater incidence of coordination between columellae and supratectal elements, implying a derivation from clavi.
Grana and verrucae occur on the floor ("foot- layer") of the lumina in all three taxa (i.e. S. barghoornii, S. pocockii and L. bulbiferum), but not in all similar crotonoid monosulcates from the Early Cretaceous (cf. Hughes et al., 1979; Penny, 1986). Whether these sculptural elements are homologues of columellae or supratectal elements is uncertain.
Twelve of the 33 grains survived sample preparation as clumps because they are physically joined to one another, indicating that they were clumped at the time of fossilization. For such large specimens to be fossilized in aggregates of two or three grains implies that they were transported to the site of deposition on a substrate that would keep them together, such as a dehisced pollen sac or plant part, or the hairy body of an insect. No evidence of large fragments of plant cuticles belonging to a possible pollen sac or leaf was found in the preparation (whole chunks of rock were digested in acids), and insect cutin rarely preserves.
The holotype (along with eight other specimens) no longer exists, having disintegrated on its slide since this manuscript was completed (see General discussion). A para type specimen (USNM455218) in good shape and well preserved was designated to replace the holotype (USNM455219), which is retained by photograph (Plate II, 1) because its dimensions and shape coincide with the average of all specimens of this taxon.
PLATE I
Selected dinoflagellate taxa from sample 1053. Magnifications to scale. Bar at bottom of plate represents 40 micrometers.
1. Gonyaulacysta centriconnata Riding 1983.
2. Gonyaulacysta jurassica adecta Sarjeant 1982, var. longicornis (Deflandre) Sarjeant 1982.
3. Stephanelytron caytonense Sarjeant, Emend. Stover, Sarjeant, and Drugg 1977. Ventral surface with single
large antapical corona.
4. Systematophora areolata Klement 1960.
5. Korystocysta pachyderma (Deflandre) Woollam 1983. Internal view of dorsal surface. Intratabular ridges
evident in postcingular plates 3"' and 4"' .
6. Scriniodinium galeritum reticulatus Klement 1960. Optical section.
7. Korystocysta norrissii (Pocock) Woollam 1983. (7a) External view of obliquely oriented ventral surface.
Apical series in focus, as well as intratabular ridges in postcingular series. (7b) Internal view of obliquely
oriented dorsal surface. Pallepicystal archeopyle evident. Note prominent intratabular ridges in postcingular
series.PLATE II (see p.278)
Stellatopollis pocockii, LM (1,3-6), and extant Lilium bulbiferum, LM (2).
1. S. pocockii n. sp., holotype, showing monosulcus; two focal levels spliced together because flattened grain
bent: 92 x 57 um. USNM455219 (dissolved as of 6-4-91; see paratype USNM455218).
2. L. bulbiferum, extant, showing a decrease in size of crotonoid sculpture only along sulcus margin: 81 x 61 um.
3. S. pocockii n.sp., the smallest specimen found: 69 x 45 um. USNM455220.
4. S. pocockii n. sp., focused on sculpture: 102 x 84 um.
5. S. pocockii n. sp., showing slighty corroded exine: 90 x 45 um.
6. S. pocockii n. sp., showing severely corroded exine: 87 x 49 um.
PLATE III (see p.279)Extant Lilium bulbiferum, LM (I) and Stellatopollis pocockii, LM (2).
1. L. bulbiferum, extant, showing clumping of pollen in preparation; three grains: 99, 81 and 75 um long.
2. S. pocockii n. sp., showing clumping prior to fossilization; note variation in preservation of sculpture and
decrease in size of lumina along sulcus margin; three grains: 105 x 45 um, 90 x 75 um, 81 x 40 um.PLATE IV (see p.280)
Stellatopollis pocockii, LM.
1. Two adhered specimens fused together by corrosion during fossilization; note possible endexine preserved in
specimen on left; two grains: 93 x 78 utm, 81 x 63 um. USNM455211.
2. Three adhered specimens fused together, possibly in tetrad orientation; two focal levels spliced together; three
grains: 93 x 51 um, 90 x 51 um, 85 x 48 um. USNM455212.PLATE V (see p.281)
Stellatopollis pocockii, SEM; showing variation in sculpture.
1. Proximal view, showing reduction in size of lumina at apices of grain, as is typical of monocot pollen: 83 x
47 um.
2. SEM enlargement of specimen in fig.l, showing crotonoid sculpture perched on top of narrow muri and short
columella positioned directly beneath some supratectal elements (arrows).
3. Lateral view, with sulcus along side with finer reticulum; note slightly coarser sculpture than in fig.l: 80 x 40 um.
4. SEM enlargement of specimen in fig.3, showing well-developed triangular elements at triple junctions of muri,
variation in width or columellae (arrows), and their offset relationship relative to supratectal elements.
Genus Multimarginites Germeraad, Hopping et Muller 1968 emend.
Type species: Multimarginites vanderhammenii Germeraad, Hopping et Muller 1968.
Emended diagnosis: Spherical, di- or triporate, multimarginate but fundamentally polyplicate; in diporate grains ridges and furrows crossing perpendicular on opposite sides of grain (90 degrees rotated bilateral symmetrical); in triporate grains furrows always normally oriented (parallel); pores covered by sexinal caps (possibly modified ridges); wall thick, tectum (ridges) perforate or foveolate-fossulate.
Remarks: The morphorogy of two-porate and 90o rotated pollen of M. vanderhammenii from Tertiary sediments of Venezuela has been considered essentially identical to that of modern Bravaisia integerrima, B. tubiflora, Sanchezia klugii and Trichanthera gigantea of the tribe Trichanthereae (e.g. Plate VIII, 6,7) (Germeraad et al., 1968; Vasanthy and Pocock, 1986). These authors interpret acanthaceous grains as colpate, with the two or three "colpi" containing centrally-positioned pores. However, there is also no srructural or apparent functional distinction between the "colpi" of Vasanthy and Pocock (1986) and the adjacent furrows, other than incidental differences. (i.e., the presence of symmetrical pores, the spacing of ridges and the presence of sculpture on the exposed nexine). Germination is interpreted as via pores, not colpi. Therefore, these grains are instead interpreted as porate and polyplicate (with only the tectum, but not the columellae, being-discontinuous between the ridges). Furrows, however, can function as sites of germination (take for example the polyplicate morphology of modern Spathiphyllum pollen: Araceae), and later be modified into colpi (cf. Cornet, 1989a). Furrows may have been retained in multimarginate acanthaceous pollen because no true colpi were present initially to accommodate exine expansion during harmomegathis.
For detailed descriptions and illustrations of Acanthaceae pollen with rotated symmetry and diporate apertures see Vasanthy and Pocock (1986). Their illustrations will be needed in order to appreciate the remarkable similarity between acanthaceous pollen and the taxon described below.
Multimarginites sp. A (Plate VI, 3-6; Plate VII, 2-4)
Description: Spherical, diporate, polyplicate; pores opposite and centrally positioned within their furrows ("colpi"); ridges and furrows crossing perpendicular on opposites sides of grain (90o rotated bilateral symmetrical); exine thick, two layered with continuous nexine or footlayer beneath tectum (ridges); columellae small, short and numerous; columellae aligned along margins of ridges, but grouped and partially fused to form rings centered under axes of ridges; rings typically with hollow centers, sometimes incomplete; ridges (tectum) perforate and foveolate-fossulate, with pores being covered or crossed by expanded exinal caps, which resemble auriculae or modified (convex) ridges; within furrows (between ridges) nexine apparently sculptured with free-standing columellae or grana (i.e. semitectate).
Dimensions (3 specimens): 87-138 um in diameter.
Remarks: Multimarginites sp. A is not given a formal specific epithet because only three specimens were found: One of them (Plate VI, 4-6) completely dissolved, while another (Plate VII, 2-4) became enlarged and translucent since they were preserved as single grain mounts and photographed ten years ago. The third specimen (Plate VI, 3) is a partial grain and was discovered in the original residue containing Stellatopollis pocockii n. sp. in 1987. The photographs, however, preserve all important information for their description and comparIson.
Multimarginites sp.A compares very closely with the pollen of extant Sanchezia and Bravaisia (Vasanthy and Pocock, 1986) in its overall shape, size, wall structure and apertures. Its ridges are rotated on opposite sides of the grain as in the extant species, and columellae are organized into circular structures along the axes of the ridges (Plate VI, 5,6; Plate VII, 2,3: white arrows), although not as frequently. Two pores exist on opposite sides of the grain and can be easily identified by their auriculate caps of protruding exine (Plate VI, 4, 6; Plate VII, 2: circles). Only the specimen in Plate VI, 4-6 shows both pores clearly (Plate VI, 5: white arrows), while one pore is obscured by folding in Plate VII, 2-4. Furthermore, the pores are located midway between the converging ridges on each side, a condition peculiar to the Acanthaceae (Vasanthy and Pocock, 1986).
The only minor differences between Multimarginites sp. A and the pollen of Sanchezia and Bravaisia are the somewhat fossulate shape of the lumina and muri and the less-well developed circular columellar structures underlying the ridges. These differences may indicate a more primitive organization and sculpture, or they may fall within the range of variation for these characters within the Acanthaceae.
Comparison with Cornetipollis reticulata
Pocock and Vasanthy (1988) described a new late Carnian (Late Triassic) pollen taxon from the Chinle Formation of Arizona, USA and compared it with the late Carnian Equisetosporites chinleanus from the same formation, and with the pollen of fossil and extant Tricanthereae of the Acanthaceae (also Vasanthy et al., 1990). They suggested. that Cornetipollis (Plate VIII, 1,2, 4,5) may represent a possible progenitor of the tribe Tricanthereae, with similarities and differences indicative of partial exine homology and primitive pollen simplicity for Cornetipollis. Specimens of C. reticulata from the Chinle Formation rarely show rotated ridge symmetry as in the Trichanthereae, but Cornetipollis cf. C. reticulata (Plate VIII, 1,2, 4,5; Pocock and Vasanthy, 1988: Plate VIII, 1) from the early-middle Carnian Richmond basin of Virginia (Cornet, 1989a) possesses partial to complete ridge rotation on opposite sides of the grain in nearly all the specimens studied by Cornet. However, the Oxfordian specimens also show two additional characters of the Acanthaceae: (1) columellae organized into circular structures; (2) two pores situated on opposite sides of the grain and overlain by protruding exinal caps or supra-oral flaps (sensu Vasanthy et al., 1990).
PLATE VI
Extant Sanchezia nobilis, LM (1,2) and Multimarginites sp. A, LM (3-6).
1. S. nobilis, extant, showing rotated symmetry and perforated ridges, with bulging pore at top: 90 11m diam.
2. Same specimen as in fig.l, focused on columellar structure beneath ridges; note circular columellae in rows
along ridge axes.
3. M. sp. A, 130 um long fragment, focused on columellar structure beneath ridges; note circular columellae
(white arrows), which are not as well developed as in extant species. USNM455214.
4-6. M. sp. A, showing three focal levels through grain: auriculate pores circled in figs.4 and 6; porate apertures
in focus in fig.5 (white arrows), while circular columellae in focus in fig.6 (white arrows): 90 x 78 um.
USNM455214.PLATE VII (see p.286)
Extant Sanchezia nobilis, LM (I) and Multimarginites sp. A, LM (2-4).
1. S. nobilis, extant, focused on one auriculate pore and columellar structure: 102 um diam.
2-4. M. sp. A, showing three focal levels through grain, which was folded during mounting; note corroded
condition of exine on one side and strong degree of compression, which permits botn sides to be visible in one
focal plane; auriculate pore on one side circled, the opposing pore concealed by fold; circular columellar
structures better developed (white arrows): 138 x 87 um. USNM455215.PLATE VIII (see p.287)
Cornetipollis cf. reticulata Pocock and Vasanthy, SEM and LM and extant Sanchezia nobilis, LM.
1. C. cf. reticulata, palynoflorule VB4, early Carnian, Richmond basin, VA, U.S.A.; SEM showing ridges
rotated 90° on opposite side of grain and scalariform-like sculpture: 108 um in diameter.
2. C. cf. reticulata, palynoflorule VB4, early Carnian, Richmond basin, VA, U.S.A.; LM focused on scalariform
muri of ridges: 114 x 111 um.
3. S. nobilis, extant, SEM focused on columellae exposed in furrow between perforated ridges.
4. SEM enlargement of fig. 1, showing detail of sculpture and grana between ridges; note columellae visible
beneath some scalariform muri.
5. SEM enlargement of fig. 1, showing detail of sculpture and grana between ridges.
6. S. nobilis, extant, SEM showing rotated symmetry of ridges and opposing positions of auriculate pores: pollen of this species ranges. from 93 um to 126 um in diameter (av. 110 um).
7. S. nobilis, extant, SEM of one symmetrical side showing the influence of one large pore in modifying the
reticulate-polyplicate sexine.
Although the unusual di-triporate ridged pollen of some Acanthaceae is usually thought to be derived from the tricolporate forms that are wide-spread in other dicotyledons, an alternative possibility involving independent derivation from an inaperturate or polyplicate ("ephedroid") type has not been fully explored. Would the occurrence of "ephedroid" characters within the Acanthaceae and related Scrophulariaceae and Bignoniaceae (e.g. Saritaea: Buurman, 1977) be "anticipated" if these families were derived from ancestors that produced polyplicate pollen instead of monosulcate pollen? If Multimarginites sp. A is closely related to the Tricanthereae of the Acanthaceae, would its early occurrence imply that the phylogenetic lineage leading to the Acanthaceae separated from gymnosperms and angiosperms unrelated to the Acanthaceae by the early Late Jurassic (cf. Martin et al., 1989; Troitsky et al., 1991)? It should be noted, however, that such a scenario strongly conflicts with currently accepted interpretations based on living angiosperms that the Acanthaceae are a relatively derived group within non-Magnoliid dicotyledons.
General discussion
Stellatopollis pocockii n. sp. and Multimarginites sp. A resemble the pollen of angiosperms in having a columellate exine and a reticulate or perforate tectum. Based on our current understanding of exine morphology and function in angiosperm pollen (e.g. Shivanna and Johri, 1985), reticulate exine sculpture supported by collumellae implies sporophytic (stigmatic) germination and an intra-specific self-incompatibility system (SSI) (Zavada and Taylor, 1986). The type of crotonoid sculpture present on S. pocockii n. sp. is identical or similar to that of various angiosperm-like crotonoid monosulcate taxa described from the Barremian (e.g. Hughes et al., 1979; Penny, 1986), while Multimarginites sp. A, in addition to resembling some Acanthaceae pollen, also resembles some species of Cornetipollis and Equisetosporites from the Mesozoic (cf. Cornet, 1981; Vasanthy and Pocock 1986; Pocock and Vasanthy, 1988; Vasanthy et al., 1990).
About nine of the 33 specimens of Stellatopollis pocockii n. sp. (including the holotype) and one of the three Multimarginites sp. A did not survive their eleven years as single grain mounts, while several of the grains that did survive became enlarged and more translucent. Apparently they were over-oxidized during preparation, and dissolved or disintegrated in the glycerol-water mounting solution. In contrast, single grain mounts of gymnosperm pollen, cryptogam spores and dinoflagellates from the same preparations and mounted in a similar manner all survived undamaged. The hyper-sensitivity of Oxfordian angiosperm-like pollen to oxidation may be significant with regard to preservation and may account in part for their poor fossil record in the Jurassic.
Phylogenetic significance and relevant pre-Cretaceous fossils
During the 1970's a deluge of new information was published on the evolution of Early Cretaceous angiosperms (e.g. Doyle,. 1973; Wolfe et al., 1975; Hickey and Doyle, 1977; Doyle and Hickey, 1976), but such discoveries have so far failed to narrow the morphological gap between angiosperms and gymnosperms (cf. Walker et al., 1983; Walker and Walker, 1984; Dilcher and Crane, 1984; Crane et al., 1986; Dilcher and Kovach, 1986; Friis et al., 1986; Friis and Crepet, 1987; Taylor and Hickey, 1990). Walker and Walker (1984: p.516) concluded from their study of Early Cretaceous pollen that the earliest stage (Stage I) of angiosperm evolution has yet to be recognized. Angiosperm-like fossils become fewer and harder to find below the Albian and seem to disappear below the Barremian or upper Hauterivian (Doyle et al., 1977; Doyle, 1978, 1983; Retallack and Dilcher, 1981, 1986; Brenner, 1984, 1987; Crane, 1987; Lidgard and Crane, 1988). Angiosperm-like pollen has been detected in the Hauterivian in rocks slightly older than those containing the oldest accepted angiosperm leaves (Brenner, 1984, 1990). Chris Hill (pers. commun., 1980) provided Cornet with photographs of an enigmatic seed plant from the Valanginian of Spain (i.e. Montsechia vidalli) that appears to have sessile stamens with paired anthers and axillary ascidiate carpels (on different branch systems), but has small curved needle-like-'leaves subtending the carpels. Consequently, it is difficult, to know whether the apparent scarcity of angiosperm remains below the Barremian is due to evolution, preservation, sampling error, recognition, limited exposure of Neocomian strata, or some combination of these factors.
Traverse (1988), Pocock and Vasanthy (1988), Cornet (1979, 1980, 1981, ~1989a), Cornet and Olsen (1990) and Doyle and Hotton (1991) illustrated and/or reported a diversity of pollen from the Late Triassic of North America that exhibit angiosperm cparacteristics, particularly col- umellae, sexine architecture and a non-laminated nexine. Cornet (1986, 1989b) described the reproductive structures, seeds, pollen, leaf venat!on and cuticular characteristics, stem and root anatomy, habit and habitat of the enigmatic Late Triassic. Sanmiguelia lewisii, concluding that this monocot-like plant (Tidwell et al., 1977) may be a very primitive dicot below the level of extant Magnoliidae and close to the evolutionary branch between monocots and dicots. Cornet (1987, 1989b), based on the. discovery of additional well-preserved flowers and reproductive organs (i.e. Axelrodia), provided evidence that Sanmiguelia had a closed carpel with apical stigma, and an ovary containing a pair of basal anatropous ovules and possible pollen-tube, transmission tissue. He also measured a series of carpels and their ovules preserved at different stages of development, and found evidence for an initial delay in ovule development relative to carpel growth, followed by exponential ovule growth. He compared this growth pattern to angiosperms and found a similar pattern in angiosperms that was related to the timing of fertilization.
The early to middle Carnian Crinopolles Group of monocot-like pollen from the Richmond basin, Virginia (Cornet, 1989a; Cornet and Olsen, 1990) demonstrates that numerous palynological characters thought to be typical and diagnostic of monocots (Doyle, 1973; Walker and Walker, 1984) appeared very early in the Mesozoic, possibly near the time of angiosperm origin (Crane, 1985; Doyle and Donoghue, 1986: p.384). If Sanmiguelia is an angiosperm or angiosperm ancestor, the existence of a possible "dicot" with monocot-like leaves and inflorescences in the Triassic (Cornet, 1986, 1989b) could support Burger's (1981) contention that monocot characters" played a significant role in early angiosperm morp.hology. Consequently, the presence of pollen in the Oxfordian that closely resembles the pollen of extant Lilium bulbiferum is consistent with the presence of monocot-like pollen at the beginning of the angiosperm radiation in the Cretaceous (Doyle, 1973; Doyle et al., 1977; Walker and Walker, 1984, 1986), and with monocot-like pollen in the Late Triassic (Cornet, 1989a).
Other putative angiosperm grains from the Jurassic and Early Cretaceous have been assigned to the genus, Clavatipollenites, which is thought to be representative of the Chloranthaceae or primitive dicots based on later studies (Walker and Walker, 1984; Friis et al., 1986; Endress, 1987; Taylor and Hickey, 1990). Clavatipollenites has been reported. ft:°m the Early, Middle and Late Jurassic. (Schultz, 1967; Horowitz, 1970; Lund, 1977; Pocock, 1978), but a detailed study of exinal sculpture and structure has not been undertaken. A small monosulcate resembling Clavatipollenites does occur in the Jurassic (also present in sample 1053: Table III; USNM455225), but it possesses a distinctive alveolar exine structure similar to that of cycad pollen (Audran and Masure, 1977), and may have been mistakenly identified as C. hughesii (e.g. Vigran and Thusu, 1975). Clavatipollenites sp. is also reported here from the Oxfordian (see Table III). This species (not illustrated) differs from C. hughesii in possessing larger lumina along the equatorial sides of the grain. It possesses a minute connate supratectal structure like that of Clavatipollenites (Walker and Walker, 1984).
Large pollen size
The extremely large size of the specimens, greater than most other Mesozoic pollen grains, raises questions about their mode of dispersal, particularly since most of the dominant pollen grains in palynoflorule 1053 (Tables II, III and IV) are under 50 um in length or diameter. Large bisaccate pollen dominates some palynoflorules of Norian (Late Triassic) age from the Newark Supergroup, Eastern North America (cf. Cornet, 1977; Cornet and Olsen, 1985, 1990): for example, most bisaccate pollen from the upper Perkasie Member of the Passaic Formation, Newark basin, New Jersey (Olsen, 1989) falls between 50 um and 156 um in overall length (average 71.6 um for 110 grains) and has corpus or body size that range from 41 um to 136 um in diameter or maximum dimension. Some of the largest saccate grains belong to (Colpectopollis cf. C. ellipsoideus. This species is common, ranges from 103 um to 156 um in length, and has low-relief uninflated sacci that reduce little the density of the grain. Because the average size of Perkasie bisaccates is so high, higher average wind velocities may have existed during Perkasie time, requiring higher grain weights or densities to achieve necessary terminal settling velocities for capture by receptive female organs (cf. Crane, 1986). Even though the majority of Jurassic pollen was probably wind dispersed, a similar explanation of higher wind velocities for Stellatopollis pocockii n. sp. (av. 92 um in length) arid Multimarginites sp. A (av. 114 um in diameter) is more difficult to implicate because of the much smaller average size of pollen in the Oxfordian (estimated at about 40 um for palynoflorule 1053). Wind pollinated plants usually produce large amounts of pollen, although such pollen could be rare if the parent
plants were located a great distance from sites of pollen deposition or if parent plant density was very low.If it is unlikely that Stellatopollis and Multimarginites were wind dispersed, could they have been insect dispersed? The fact that we could not duplicate these results in samples above and below the productive one (sample 1053) implies that their occurrence is fortuitous and unlikely to be easily repeated elsewhere for the Upper Jurassic. Is the occurrence of both S. pocockii n. sp. and M. sp. A only in sample 1053 and of S. pocockii n. sp. in clumps mere coincidence, or could this association imply the presence of a fossil insect in the sample? There was evidence of small darkly stained impressions (not plant cuticle) on surfaces of some of the rock fragments in the sample envelope (q.v. photos provided with slide collection), and it is tempting to speculate that they may have belonged to such an insect fossil. We therefore suggest that paleoentomologists should examine specimens of fossilized insects from the Upper Jurassic for pollen that may have been clinging to their exoskeletons at the time of burial.
Age ranges of various insect taxa have been used to imply a correlation between first occurrences of insect groups known to pollinate angiosperms and the appearance of flowers with significant, evolutionary innovations in the Cretaceous (Crepet and Friis, 1987; Friis and Crepet, 1987). Coleoptera were probably the major pollina- tors of Early Cretaceous angiosperms, "but it is also possible that early magnoliids were generalists and that flies, micropterigid moths, sawflies and even sphecid wasps participated in their pollination biology." (Crepet and Friis, 1987: p.187). Coleoptera and Diptera are known from the Carnian (Late Triassic), in particular possible Tipulidae (crane flies) and Bibionidae (march flies) (Olsen et al., 1978), while a putative primitive lepidopteran (i.e. Archaeolepis) has been described from the Early Jurassic (early Siriemurian of Charmouth, Dorset, England: Whalley, 1985). A more advanced lepidopteran with scales on both wings (i.e. Eolepidopterix) was found in the late Middle or early Late Jurassic (Uda River in Trapsbaykalia: Rasnitsyn, 1984), establishing the potential for a long period of coevolution between the Lepidoptera and their moden angiospermous hosts (Shields, ]988). Extant Sanchezia and Lilium have flag and tube blossom types that are commonly pollinated by Lepidoptera (Faegri and van der Pijl, ] 979: 97).
Conclusion
The early Oxfordian age of the angiosperm-like pollen described in this paper is confirmed by dinoflagellate biostratigraphy. The presence of Stellatopollis and a crotonoid sculpture in the Oxfordian becomes less remarkable when compared to the prevalence of similar pollen characters in the earliest Cretaceous angiosperm pollen record of southern England (e.g. Hughes et al., 1979). The presence of Multimarginites and diporate pollen in the Oxfordian becomes less remarkable when compared to the antiquity of the probable progenitor morphotypes, Cornetipollis and Equisetosporites (see Pocock and Vasanthy, 1988). An older occurrence of porate pollen does not affect the significance of tricolpate pollen in the Early Cretaceous record, but may imply an older and separate evolutionary history for the acanthoid morphotype and its phylogenetic lineage (cf. Pocock and Vasanthy, 1988; Martin et al., 1989; Troitsky et al., 1991). Consequently, the earlier appearance of porate angiosperm-like pollen may be unrelated to Doyle's (1977) stratigraphic evidence for tricolpate to porate pollen evolution within the Magyoliidae and related higher subclasses.
The pollen grains described in this paper contribute to other slowly accumulating evidence that implies angiosperms separated from the ancestors of the Bennettitales and Gnetales before the Carnian in the Triassic or Permian (q.v. Axelrod, 1952, 1961; cf. Crane, 1985; Doyle and Donoghue, 1987; Martin et al., 1989; Troitsky et al., 1991). Angiosperm-like pollen as distinctive as Stellatopollis and Multimarginites in the early Oxfordian implies that angiosperms existed well before the Hauterivian. The complex reasons for the implied delay in angiosperm radiation remain to be determined (Taylor, 1988).
Acknowledgements
We wish to thank the late Gulf Research & Developmen! Co., Houston, TX (merged with Chevron Corp.) and Chevron Corporation for permission in 1980 and 1990, respectively, to publish this study. We also thank Gulf Oil Corporation for the use of their materials, facilities, and equipment, and Thomas D. Shell formerly at Gulf R.& D. Co. for the SEM micrographs of Stellatopollis pocockii n. sp. (Plate V). We acknowledge U.S. National Science Foundation Grant BSR 87-17707 to P.E. Olsen for support to B. Cornet in 1988-1989; and U.S. National Science Foundation Grant EAR-8903522 to D. Habib at Queens College for support of his contribution. We also wish to acknowledge the late G. Thanikaimoni for specimens of S. nobilis, James W. Walker and Audrey G. Walker for the SEM microlgraphs of Cornetipollis cf. reticulata and Sanchezia nobilis (Plate VIII) and Lewis Stover (retired, Exxon Corp.) for allowing B. Cornet to use his microscope. We thank E. Parascandolo and R. van Pelt for their technical assistance to D. Habib. Cornet acknowledges and greatly appreciates the encouragement and support of the late George R. Fournier during a time when talk of pre-Cretaceous angiosperms was considered tantamount to heresy.
This paper is dedicated to the memory of my wife, Bonnie Lee Cornet, who passed away on 12 January 1991 at age 40. If it were not for her support this manuscript might never have gotten to the publisher.
Addendum
In May of 1991 a well-preserved dicot-like leaf, 3.2 cm long and 1.3 cm wide, was found in late Carnian age, deposits (Cow Branch Fm.) of the Dan River basin, North Carolina by Paul E. Olsen and Annika Johansson (Solite quarry: Olsen et al., 1978). This small oval leaf is distinctive in having pinnate venation (15 secondaries), festoon brochidromous secondary and tertiary venation, a very regular reticulate venation inside the brochidromous loops and a dendritic dichotomous venation along the margin of the leaf. The base of the leaf and leaf tip are incomplete. The lowermost left margin curves abruptly towards the primary vein, but terminates just before meeting the primary. The lamina on the lowermost right side is missing, while the primary vein curves gently towards the right in the lower half of the leaf. The secondary veins on the lower left side are slightly expanded or extended compared to those on the right, with the addition of a transverse tertiary vein internal to each of two extended secondary loops. The expanded lower left side of the leaf and the curved primary give the leaf a distinct asymmetry. This asymmetry, together with the absence of a lower right margin, implies that this specimen may represent only the lateral lobe of a larger three-lobed palmately veined "Platinoid" leaf (cf. Hickey and Doyle, 1977).
The venation of this leaf is more derived than that of any known fern (e.g. Clathropteris, Polypodium) or pteridosperm (e.g. Furcula, Yabeiella), and is at a comparable level of evolution (for angiosperms) as the diverse, multiaperturate, reticulate-columellate angiosperm-like pollen documented by Cornet (1989) for the Carnian of the Newark Supergroup. This leaf was probably derived from the mountainous terrain that undoubtedly existed to the west of the Dan River basin (westernmost side of the Newark Supergroup rift zone) (Olsen et al., 1978). The geographical position of the Dan River basin at the time of formation was equatorial based on Milankovitch lacustrine cycle analysis (Olsen, in press) and paleo-pole position (Witte et al., in press). A manuscript is in preparation.
References
Audran, J.-C. and Masure, E., 1977. Contribution a la connaissance de la composition des sporodermes chex les Cycladales (Prespermaphytes). Etude en microscopie electronique transmission (M.E.T.) et a balayage (M.E.B.). Palaeontographica, 162B: 115-158.
Axelrod, D.I., 1952. A theory of angiosperm evolution. Evolution.. 6(1): 29-60.
Axelrod, D.I., 1961. How old are the angiosperms? Am. J. Sci., 259: 447-459.
Brenner, G.J., 1984. Late Hauterivian angiosperm pollen from the Helez Formation, Israel. Sixth Int. Palynol. Conf., Calgary, Abstr., p.15.
Brenner, G.J., 1987. Paleotropical evolution of the Magnoliidae, in the Lower Cretaceous of northern Gondwana. Am. J. Bot., 74: Abstract 232, p.677.
Brenner, G.J., 1990. An evolutionary model of angiosperm pollen evolution based on fossil angiosperm pollen from the Hauterivian of Israel. Am. J. Bot., 77: Abstract 211, p.82.
Burger, W. C:, 1981. Heresy revived: The monocot theory of angiosperm origin. Evol. Theory, 5: 189-225.
Buurman, J., 1977. Contribution to the polymorphology of the Bignoniaceae, with special reference to the tricolpate type. Pollen Spores, 19(4): 447-519.
Cornet, B., 1977. The palynostratigraphy and age of the Newark Supergroup. Ph.D. diss., Pennsylvania State Univ., 505 pp.
Cornet, B., 1979. Angiosperm-like pollen with tectate-columellate wall structure from the Upper Triassic (and Jurassic) of the Newark Supergroup, USA. Palynology, 3: 281-282.
Cornet, B., 1980. Tropical Late Triassic monosulcate and polysulcate angiospermid pollen and their morphological relationship with associated auriculate polyplicate pollen. 5th Int. Palynol. Conf., Cambridge (1980), p.91.
Cornet, B., 1981. Recognition of pre-Cretaceous angiosperm pollen and its relationship to fossil polyplicate pollen. Palynology, 5: Abstr., pp.212-213.
Cornet, B., 1986. The reproductive structures and leaf venation of a Late Triassic angiosperm, Sanmiguelia lewisii. Evol. Theory, 7(5): 231-309.
Cornet, B., 1987. Further evidence for the reproductive morphology of Sanmiguelia lewisii and its bearing on angiosperm ancestry. Am. J. Bot., 74: Abstract 237, p.680.
Cornet, B., 1989a. Late Triassic angiosperm-like pollen from the Richmond rift basin of Virginia, USA. Palaeontographica, 213B: 37-87.
Cornet, B., 1989b. The reproductive morphology and biology of Sanmiguelia lewisii, and its bearing on angiosperm evolution in the Late Triassic. Evol. Trends Plants, 3(1): 25-51.
Cornet, B., and Olsen, P.E., 1985. A summary of the biostratigraphy of the Newark Supergroup of eastern North America with comments on early Mesozoic provinciality. In: R. Weber (Editor), III Congresso Latinoamericano de Paleontologia. Mexico. Simposio Sobre Floras del Triasico Tardio, su Fitogeografia y Paleoecologia, Memoria, pp. 67-81.
Cornet, .B. and Olsen, P.E., 1990. Early to middle tarnian (Triassic) flora and fauna of the Richmond and Taylorsville basins, Virginia and Maryland, USA. Va. Mus. Nat. Hist., Guidebook No. I, Martinsville, 87 pp.
Couper, R.A., 1958. British Mesozoic microspores and pollen grains, a systematic and stratigraphic study. Palaeontographica, 103B: 75-179.
Crane, P.R., 1985. Phylogenetic analysis of seed plants and the origin of angiosperms. Ann. Mo. Bot. Gard., 72: 716-793.
Crane, P:R.., 1986. Form and function in wind dispersed pollen. In: S. Blackmore and I.K. Ferguson (Editors). Pollen and Spores: Form and Function. Academic Press, London (Linn. Soc. London), pp.179-202.
Crane, P.R., 1987. Vegetational consequences of the angiosperm diversification. In: E.M. Friis, W.G. Chaloner and P.R. Crane (Editors), The Origins of Angiosperms and their Biological Consequences. Cambridge Univ. Press, Cambridge, pp.107:144.
Crane, P.R., Friis, E.M. and Pedersen, K.R., 1986. Lower Cretaceous angiosperm flowers: fossil evidence on early radiation of dicotyledons. Science, 232: 852-854.
Crane, P.R., 1988. Review of Cornet, B., The leaf venation and reproductive structures of a Late Triassic angiosperm, Sanmiguelia lewisii. Taxon, 36: 778-779.
Crepet, W.L. and Friis, E.M., 1987. The evolution of insect pollination in angiosperms. In: E.M, Friis, W.G. Chaloner and P.R. Crane (Editors), The Origins of Angiosperms and their Biological Consequences. Cambridge Univ. Press, Cambridge, pp.181-201.
Dilcher, D.L.and Crane, P.R., 1984. Archaeanthus: an early angiosperm from the Cenomanian of the western interior of North America. Ann. Mo. Bot. Gard., 71(2): 351-383.
Dilcher, 'D.L. and Kovach, W.L., 1986. Early angiosperm reproduction: Caloda delevoryana gen. et sp. nov., a new fructification from the Dakota formation (Cenomanian) of Kansas. Am. J. Bot., 73(8): 1230-1237.
Doyle, J.A., 1973. The Monocotyledons: their evolution and comparative biology. Q. Rev. Biol., 48: 399-413.
Doyle, J.A., 1977. Patterns of evolution in early angiosperms. In: A. Hallam (Editor), Patterns of Evolution. Elsevier, Amsterdam, pp.501-546.
Doyle, J.A., 1978. Origin of angiosperms. Ann. Rev. Ecol. Syst., 9: 365-392.
Doyle, J.A., 1983. Palynological evidence for Berriasian age of basal Potomac Group sediments, Crisfield well, eastern Maryland. Pollen Spores, 25: 499-530.
Doyle, J.A. and Donoghue, M.J., 1986. Seed plant phylogeny and the origin of angiosperms: an experimental cladistic approach. Bot. Rev., 52: 321-431.
Doyle, J.A. and Donoghue, M.J., 1987. The origin of angiosperms: a cladistic approach. In: E.M. Friis, W.G. Chaloner and P.R. Crane (Editors), The Origins of Angiosperms and their Biological Consequences. Cambridge Univ. Press, Cambridge, pp.17-49.
Doyle, J.A. and Hickey, L.J., 1976. Pollen and leaves from the mid-Cretaceous Potomac Group and their bearing on early angiosperm evolution. In: C.B. Beck (Editor), Origin and Early Evolution of Angiosperms. Columbia University Press, New York, pp.139-206.
Doyle, J.A. and Hotton, C.L., 1991. Diversification of early angiosperm pollen in a cladististic context. In: Blackmore and S.H. Barnes (Editors), Pollen and Spores, Syst. Assoc. Spec. Vol. 44. Clarendon, Oxford, pp. 169-195.
Doyle, J.A., Van Campo, M. and Lugardon, B., 1975. Observations on exine structure of Eucommiidites and Lower Cretaceous angiosperm pollen. Pollen Spores, 17: 430-486.
Doyle, J.A., Biens, P., Doerenkamp, A. and Jardine, S., 1977. Angiosperm pollen from the pre-Albian Lower Cretaceous of equatorial Africa. Bull, Rech. Explor.-Prod. Elf-Aquitaine, 1(2): 451-473.
Drugg, W.S., 1978. Some Jurassic dinoflagellale cysts. from England, France and Germany. Palaeontographica, 168B: 61-79.
Endress, P.K., 1987. The Chloranthaceae: reproductive structures and phylogenetic position. Bot. Jahrb. Syst., 109: 153-226.
Faegri, K. and van der Pijl, L., 1979. The Principles of Pollination Ecology. Pergamon Press, New York, 244 pp.
Filatoff, J., 1975. Jurassic palynology of the Perth basin, western Australia. Palaeontographica, 154B: 1-113.
Friis, E.M., Crane, P.R. and Pedersen, K.R, 1986. Floral evidence for Cretaceous chloranthoid angiosperms. Nature, 320 (6058): 163-164.
Friis, E.M. and Crepet, W.L., 1987. Time of appearance of floral features. In: E.M. Friis, W.G. Chaloner and P.R. Crane (Editors), The Origins of Angiosperms and their Biological Consequences. Cambridge Univ. Press, Cambridge, pp.145-179.
Germeraad, J.H., Hopping, C.A. and Muller, J., 1968. Palynology of Tertiary sediments from tropical areas. Rev. Palaeobot. Palynol., 6: 189-348.
Hickey; L.J. and Doyle, J.A., 1977. Early Cretaceous fossil evidence for angiosperm evolution. Bot. Rev., 43: 3-104.
Horowitz, A., 1970. Jurassic microflora from the northern Negev, Israel. Isr. J. Earth-Sci., 19: 153-182.
Herngreen, G.F.W. and De Boer, K.F., 1974. Palynology of Rhaetian, Liassic, and Dogger strata in the eastern Nether- lands. Geol. Mijnbouw, 53: 343-368.
Huang, T.-C., 1972. Pollen flora of Taiwan. Natl. Taiwan Univ. Bot. Dep. Press, 297 pp.
Hughes, N.F., Drewry, G.E. and Laing, J.F., 1979. Barremian earliest angiosperm pollen. Palaeontology, 22(3): 513-535.
Lidgard, S. and Crane, P.R., 1988. Quantitative analyses of the early angiosperm radiation. Nature, 331: 344-346.
Lentin, J.K. and Williams, G.L., 1989. Fossil dinoflagellates: index to genera and species, 1989 edition. Am. Assoc. Stratigr. Palynol. Contrib. Ser. No. 20, 473 pp.
Lund, J.J., 1977. Rhaetic to Lower Liassic palynology of the onshore south-eastern North Sea basin. Geol. Surv. Den., Series II (109), 129 pp.
Martin, W., Gierl, A. and Saedler, H., 1989. Molecular evidence for pre-Cretaceous angiosperm origins. Nature, 339: 46-48.
Olsen, P.E., 1989. Geology of the Newark, basin, stratigraphy. In: P.E. Olsen and P.J.W. Gore (Editors), Tectonic, Depositional, and Paleoecological History of Early Mesozoic Rift Basins, Eastern North America. Field Trip Guidebook T351. American Geophys. Union, W.D.C., pp.69-72.
Olsen, P.E., in press. Field guide to three Late Triassic tetrapod sites in Virginia and North Carolina (Culpeper, Richmond, and Dan River basins, Newark Supergroup). Workshop on early Mesozoic small tetrapods, Front Royal, Virginia. Columbia Univ. Press.
Olsen, P.E., Remington, C.L., Cornet, B. and Thomson, K.S., 1978. Cyclic change in Late Triassic lacustrine communities. Science, 201: 729-733.
Penny, J.H.J., 1986. An Early Cretaceous angiosperm pollen assemblage from Egypt. Spec. Pap. Palaeontol., 35: 1-11-134.
Pocock, S.A.J., 1978. Lowermost Jurassic spore-pollen assemblage from Canadian Arctic. Palaeobotanist, 25: 363-375.
Pocock, S.A.J. and Vasanthy, G., 1988. Cornetipollis reticulata, a new pollen with angiospermid features from Upper Triassic (Carnian) sediments of Arizona (USA), with notes on Equisetosporites. Rev. Paleobot. Palynol., 55: 337-356.
Rasnitsyn, A.P., 1984. The first find of a Jurassic butterfly. Dokl. Akad. Sci. USSR Earth Sci. Sect., 269: 174-177.
Retallack, G. and Dilcher, D.L., 1981, A coastal hypothesis for the dispersal and rise to dominance of flowering plants. In: K.J. Niklas (Editor), Paleobot. Paleoecol. Evol.: 27-77.
Retallack, G. and Dilcher, D.L., 1986. Cretaceous angiosperm invasion of North America. Cretaceous Res., 7: 227-252.
Reyre, Y., 1970. Stereoscan observations on the pollen genus Classopollis Pflug 1953. Palaeontology, 13: 303-322.
Riding, J .B., 1987. Dinoflagellate stratigraphy ef the Nettleton Bottom Borehole (Jurassic: Hettangian to Kimmeridgian), Lincolnshire, England. Proc. Yorks. Geol. Soc., 46: 231-266.
Riley, L.A. and Fenton, J.P.G., 1982. A dinocyst zonation for the Callovian to Middle Oxfordian succession (Jurassic) of northwest Europe. Palynology, 6: 193-202.
Schultz, E., 1967. Sporenpalaontologische Untersuchungen ratoliassischer Schichten in Zentralteil des Germanischen Beckens. Palaontol. Abh., Abt. B(2): 544-633.
Shields, 0., 1988. Mesozoic history and neontology of Lepidoptera in relation to Trichoptera, Mecoptera, and angiosperms. J. Paleontol., 62: 251-258.
Shivanna, K.R. and Johri, B.M., 1985. The Angiosperm Pollen, Structure and Function. Wiley, New York, 374 pp.
Srivastava, S.K., 1987. Jurassic spore-pollen assemblages from Normandy (France) and Germany. Geobios, 20(1): 5-79.
Taylor, D.W. and Hickey, L.J., 1990. An Aptian plant with attached leaves and flowers: Implications for angiosperm origin. Science, 247: 702-704.
Taylor, T.N., 1988. The origin and evolution of angiosperms. Atlas Sci.: Animal Plant Sci., pp.47-50.
Tidwell, W.D., Simper, A.D. and Thayn, G.F., 1977. Additional information concerning the controversial Triassic plant: Sanmiguelia. Palaeontographica, 163B: 143-151.
Traverse, A., 1988. Paleopalynology. Allen & Unwin Inc., Unwin Hyman, Boston, 600 pp.
Troitsky, A.V., Melekhovets, Yu.f., B;akhimova, G.M., Bobrova, V.K., Valiejo-Roman, K.M. and Antonov, A.S., 1991. Angiosperm origin and early stages of seed-plant evolution deduced from rRNA sequence comparisons. J. Mol. Evol., 32: 253-261.
Vasanthy, G. and Pocock, S.A.J., 1986. Radial through rotated symmetry of striate pollen of the Acanthaceae. Can. J. Bot., 64: 3050-3058.
Vasanthy, G., Pocock, S.A.J. and Vankatachala, B.S., 1990. A comparative pollen morphological (LM, SEM & TEM) study of the Triassic Cornetipollis reticulata and the tribe Trichanthereae of Acanthaceae. A.A.S.P. Program Abstr:, Oct. 10-13, poster session.
Vigran, J.O. and Thusu, B., 1975. Illustrations of Norwegian microfossils. Illustrations and distribution of Jurassic palynomorphs of Norway. Norwegian Council for Scientific & Industrial Research, Continental Shelf Division Publication, 65: 55 pp.
Walker, J.W., 1976. Comparative pollen morphology and phylogeny of the ranalean complex. In: C.B. Beck (Editor), The Origin and Early Evolution of Angiosperms. Columbia Univ. Press, New York, pp.241-299.
Walker, J.W., Brenner, G.J. and Walker, A.G., 1983. Winteraceous pollen in the Lower Cretaceous of Israel: early evidence of a magnolialean angiosperm family. Science, 220: 1273- 1275.
Walker, J.W. and Walker, A.G., 1984. Ultrastructure of Lower Cretaceous angiosperm pollen and the origin andk early evolution of flowering plants. Ann. Mo. Bot. Gard., 71: 464-521.
Walker, A.G. and Walker, J.W., 1986. Ultrastructure of Lower Cretaceous angiosperm pollen and early monocot evolution. Am. J. Bot., 73: 713.
Whalley, P.E.S., 1985. The systematics and palaeogeography of the Lower Jurassic insects of Dorset, England. Bull. Br. Mus; Nat. Hist. (Geol.), 39: 107-189.
Witte, W:K., Kent, D.V. and Olsen, P.E., 1991. Magnetostratigraphy and paleomagnetoc poles from Late Triassic-earliest Jurassic strata of the Newark basin. Geol. Soc. Am. Bull., 103: 1648-1662.
Wolfe, J.A., Doyle, J.A. and Page, V.M., 1975. The basis of angiosperm phylogeny: Paleobotany. Ann. Mo. Bot. Gard., 62: 801-824.
Woollam, R. and Riding, J.B., 1983. Dinoflagellate cyst zonation of the English Jurassic. Her Majesty's Stationery Service (London), Inst. Geol. Sci., Rep. 83/2, 42 pp.
Zavada, M.S. and Taylor, T.N., 1986. The role of self-incompatibility and sexual selection in the gymnosperm- angiosperm transition: a hypothesis. Am. Nat., 128: 538-550.