|
THE LEAF VENATION AND REPRODUCTIVE
STRUCTURES OF A LATE TRIASSIC ANGIOSPERM, SANMIGUELIA LEWISII
BRUCE CORNET
14222 Kimberley Ln., #411
Houston, TX 77079
Received 17 June 1986, 26 August 1986
ABSTRACT: An in situ vegetative colony of Sanmiguelia lewisii Brown with organic remains preserved is described from the Late Carnian upper Trujillo Formation of the Dockum Group in northwestern Texas. Well preserved leaves, leaf venation, stems, roots, rhizomes, and their anatomy, reproductive branch systems, their megasporophylls and microsporophylls, ovules, seeds, and pollen provide abundant new evidence to interpret the systematic affinities of this controversial Late Triassic plant. The vegetative and reproductive organs of S. lewisii emended are decribed in detail, with anatomical information included secondarily. Leaves possess four orders of poorly organized parallel venation, abundant cross veins, and apical vein fusion, and show evidence of intercalary growth. Carpel-like megasporophylls (Axelrodia burgeri Cornet gen. et sp. nov.) with apical stigma-like organs are borne individually on tertiary branches and at the ends of secondary branches in flower-like clusters on a paniculate inflorescence with sheathing cataphylls, subtended by a large spathe-like vegetative leaf. Paired sessile biloculate anther-like microsporophylls (Synangispadixis tidwellii Cornet gen. et sp. nov.) containing tectate-granular monosulcate pollen were borne in synangia along a spadix-like inflorescence, probably also subtended by a large spathe-like leaf. A. burgeri contains paired anatropous ovules enclosed in an ovary, and large bean-shaped seeds (identical to the dispersed seeds, Nemececkigone fabaforma Cornet gen. et sp. nov.) that developed late in megasporophyll development, and contain a dicotyledonous embryo with one cotyledon significantly larger than the other. Axes of S. lewisii have vesselless secondary xylem containing tracheids with helical-scalariform and circular bordered pits, uniseriate rays and vascular leaf traces that occupied giant multiseriate rays or wood gaps. The secondary xylem of the primary root system contains vessels. S. lewisii is compared with angiosperm-like fruiting structures from Carnian strata of the Richmond Basin of Virginia, and with Recent angiosperms, and is interpreted as a very primitive pre-magnoliid angiosperm close to the evolutionary branch between monocots and dicots. It is concluded that the poor pre-Cretaceous angiosperm record resulted from a combination of factors including poor preservation of delicate organs, the specialized ecology of early angiosperms, large fragile and non-deciduous leaves, and pollen that cannot be easily distinguished from gymnosperm pollen.
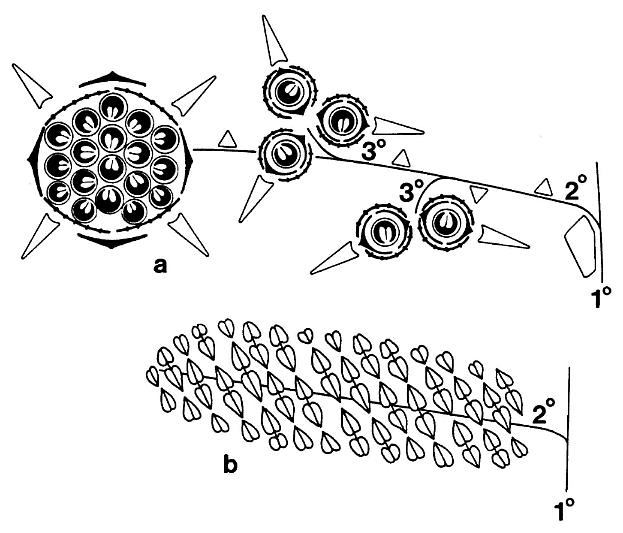
Schematic floral diagrams of Axelrodia burgeri (a) and Synangispadixis tidwellii (b) are given below; primary, secondary, and tertiary branches are labelled; the three types of bracts surrounding the megasporophylls of Axelrodia are given different symbols.
This paper is dedicated to my wife, Bonnie Lee Cornet, whose love, inspiration, and patience helped make this manuscript possible. The author wishes to acknowledge the following people for their help in finding and in collecting specimens at the Sunday Canyon locality: Drs. S.R. Ash, P.A. Murry, P.E. Olsen, and D.L. Dilcher, and the field assistance of A. Santa Luca and A.J. Litt. He also wishes to acknowledge the local geologic and paleontologic knowledge provided by P.A. Murry and P.E. Olsen, and the discussions, support, and advice of W.C. Burger and the late G.R. Fournier. The author acknowledges The Pennsylvania State University (N.S.F. grant no. GA36870 to Professor Alfred Traverse), the Philadelphia Academy of Natural Sciences, the late Gulf Research & Development Co., and Exxon Co. U.S.A. for the facilities, equipment, and materials that together made this study possible. All the drawings and reconstructions were done by Bruce Cornet.
Evolutionary Theory 7: 231-309
(September, 1986)
The editors thank W. Burger and P.R. Crane for help in evaluating this paper.
© 1986 Biology Department, The University of
Chicago
INTRODUCTION
In 1956 Brown described Sanmiguelia lewisii from the Late Triassic Dolores Formation of Colorado, and on the basis of leaf shape and venation, suggested a possible relationship with the palmae. Subsequently, the phylogenetic relationships of Sanmiguelia have been controversial, and comparisons have been made rith taxa ranging from monocotyledons to cycads, and even the Irobable arthrophyte Schizoneura (Tidwell et al., 1977). With the exception of carbonized leaves reported by Ash (1976), all previously described material of Sanmiguelia consisted of leaf mpressions in red siltstone and fine sandstone, or three-dimensional casts of stems with attached leaves (Tidwell et al., 1977).
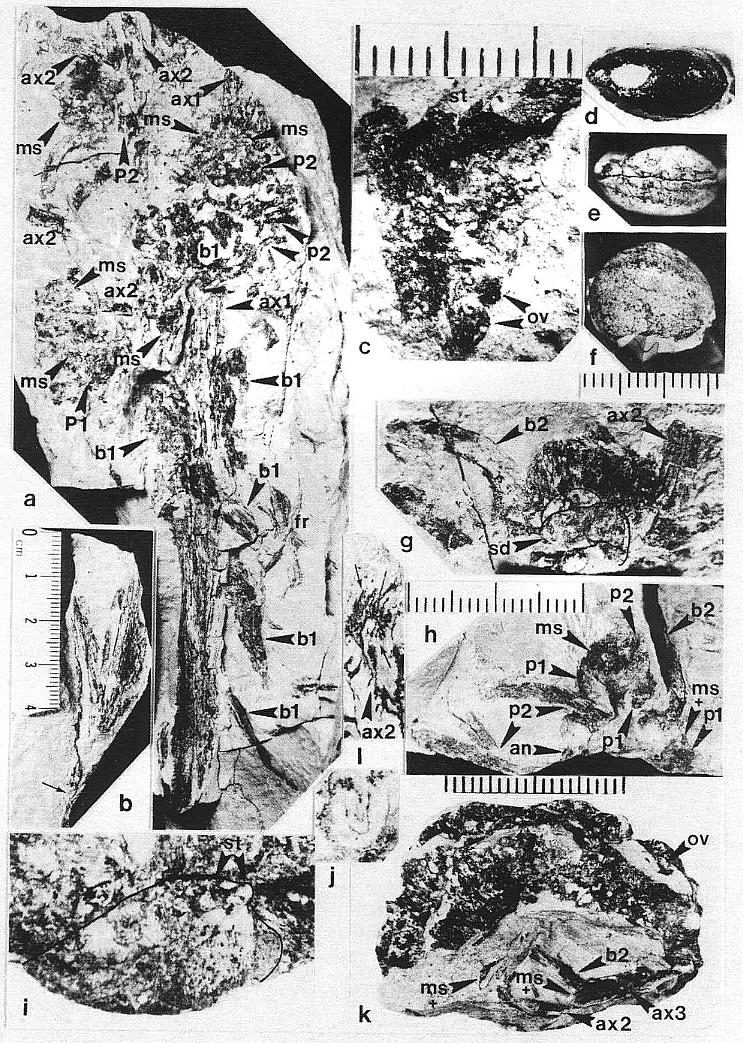
Since the specimens reported by Ash (1976) came from a previously unrecognized locality in Texas, and had indications of veins preserved between the plications, an attempt was made to recover more complete material from Ash's locality (figure 1). For three days only fragments of leaves similar to those illustrated by Ash were found, but persistence and luck eventually combined to make a story with a happy ending: As the sun began to slip below the horizon on the last planned day of the expedition, one last attempt was made. This time a swing of a pickaxe revealed a nearly complete reproductive axis (Pl. 6, fig. a). With additional excavation in the following months, an entire vegetative colony of Sanmiguelia was discovered in growth position that yielded unusually well-preserved leaves, stems, roots, wood, reproductive axes, seeds, and pollen.
This paper deals primarily with the reproductive organs and leaf venation of Sanmiguelia lewisii. Descriptions and illustrations of wood and root anatomy, additional data on leaf morphology and variation, the wall structure of in situ pollen, and a reconstruction of the entire plant will be published later. Pertinent data on anatomy, however, is briefly discussed and illustrated. All specimens described and illustrated in this paper are deposited in the paleobotanical collections of the Field Museum of Natural History, Chicago (PP).
GEOLOGIC OCCURRENCE
The Sanmiguelia specimens described here come from near the top of the Trujillo Formation of the Dockum Group of northwest Texas (figure 1). Palynoflorules from the matrix containing Sanmiguelia specimens and from nearby shales at the locality are identical to those described by Dunay and Fisher (1979) from the upper Dockum Group, and indicate a Late Carnian age. Dunay and Fisher's (1979) study includes a palynoflorule (7A) from the same Sanmiguelia locality. A diverse pollen assemblage was found clinging to some Sanmiguelia leaf cuticles; some of those palynomorphs (e.g. Succinctisporites cf. S. circumdatus Leschik, 1955) are diagnostic of the Carnian (Pl. 2, fig. f). All specimens of Sanmiguelia come from one locality along a dirt road winding down the north wall of Sunday Canyon, just west of Palo Duro Canyon State Park, in Randall County, Texas (Lat. 101° 44'; Long. 34° 50'). The strata containing Sanmiguelia occur just below a sequence of conglomerate and sandstone, and appear to represent a shallowing upwards interdistributary lake deposit on top of a paleosol. The Sanmiguelia colony is restricted to the west end of a long gray mudstone lens, which is terminated westward by a down-cutting sequence of channel sandstone with conglomerate lag at its base (figure 1). The lacustrine clam shrimp, Cyzicus sp., occurs in some of the dark-gray shale interbeds within the lake sequence.
MATERIALS, METHODS, AND OCCURRENCE
The remains of Sanmiguelia lewisii were found both in growth position and as fallen leaf-bearing axes along bedding planes (Pl. 1, fig. a; Pl. 4, fig. a). The vertical axes are preserved as pith casts surrounded either by carbonaceous residue or petrified wood (Pl. 2, figs. a and b). Small plicate leaves are attached to the lower part of some vertical axes (figure 2b), while only sheathing leaf bases are preserved on others (Pl. 2, fig. a) - no large leaves are attached. Most leaves found in the siltstone layers above the paleosol are either twisted, torn, or fragmented, probably reflecting damage during burial.
Portions of large leaves were also found oriented parallel to bedding in the shale directly above the paleosol in which the vertical axes were rooted. Both vertical and fallen (horizontal) axes had attached leaves that are morphologically identical to the leaves of Sanmiguelia lewisii (compare figure 2b with Pl. 1, figs. a-b). Some vertical axes lacking attached leaves (figure 2a) had adjacent fallen axes (e.g. Pl. 1, figs. a-b) possessing Sanmiguelia type leaves, while others had attached leaves only at their distal ends. Although some gymnospermous fossils (isolated leaves, stems, and cones) were found preserved in the paleosol, with few exceptions, Sanmiguelia, its vegetative and reproductive organs, and the remains of ferns were the only determinable fossils found above the paleosol in the sediments surrounding the upright stems.
The friable nature of the sediments presented a problem in removing specimens, which sometimes were larger than the area of the excavation. Damage invariably occurred to some large specimens, with some small pieces of rock crumbling along fractures, joints, and breaks. Several of the specimens have been carefully reconstructed in the laboratory.
Some of the specimens required degaging in order to reveal hidden parts. Most specimens, however, provided enough evidence for study and interpretation without any significant preparation. Acetate peel transfers were made of well-preserved leaves, and a JEOL SEM was used to study individual microsporophy11s and transfer preparations of aggregates of microsporophy11s from large reproductive structures. Standard palynological techniques were used to secure pollen from microsporangia, as well as cuticle fragments from megasporophylls. Such preparations of pollen and cuticle, as well as transfer peals of leaf cuticle, were studied and photographed with a Zeiss Photomicroscope with built-in camera. Photographs of the megafossils were made using both Hasselblad 500 cm and Minolta 35 mm cameras with enlargement lenses.
The reproductive organs are not described under Sanmiguelia lewisii, but are given their own binomial names, because 1) two distinctly different types of paniculate inflorescences were found, neither of which was organically connected to Sanmiguelia leaves (even though they overlapped them), 2) it is preferable that each inflorescence type have its own designated holotype and a formal diagnosis and description, 3) a taxon based on leaves suffers the risk of becoming a formgenus if the morphology of its leaves proves to be generalized within a natural genus or family, 4) the leaves at the Sunday Canyon locality, although they are indistinguishable in general form from S. lewisii, may belong to a different species, and 5) later investigators can always place these names in synonymy with S. lewisii or designate one or more of the names as taxa distinct from S. lewisii. The dispersed seeds are given a separate name even though identical seeds were found inside a mature megasporophyll, because dispersed pollen is given a separate name even when its origin is known. A third type of reproductive structure resembling a part of one of the paniculate inflorescences was found attached to stems bearing Sanmiguelia leaves, but this third type is interpreted here only as supporting evidence that the paniculate inflorescences belong to Sanmiguelia.
SYSTEMATIC DESCRIPTIONS
Sanmiguelia Brown emend. gen.
TYPE SPECIES: Sanmiguelia lewisii Brown
EMENDED DIAGNOSIS: Primary axis or stem simple, round in transverse section, erect. Narrow elongate secondary branches originate at low angles from primary axis and diverge outwards. Small to large leaves borne singly on primary axis in a loose to crowded spiral. Very small leaves and bracts borne singly on secondary axis in a loose spiral. Leaves broadly oval or el- liptic in shape, widest in the middle, tapering to an acute or acuminate apex (tip frequently missing); narrow below, but not petiolate and attached by a broad transverse base, which clasps and decurrently sheathes the stem.
Leaves with up to four orders of parallel venation. All vein orders anastomose and bifurcate: Primary veins rarely, secondary veins occasionally, tertiary veins commonly, and quaternary veins abundantly. Tertiary and quaternary veins often
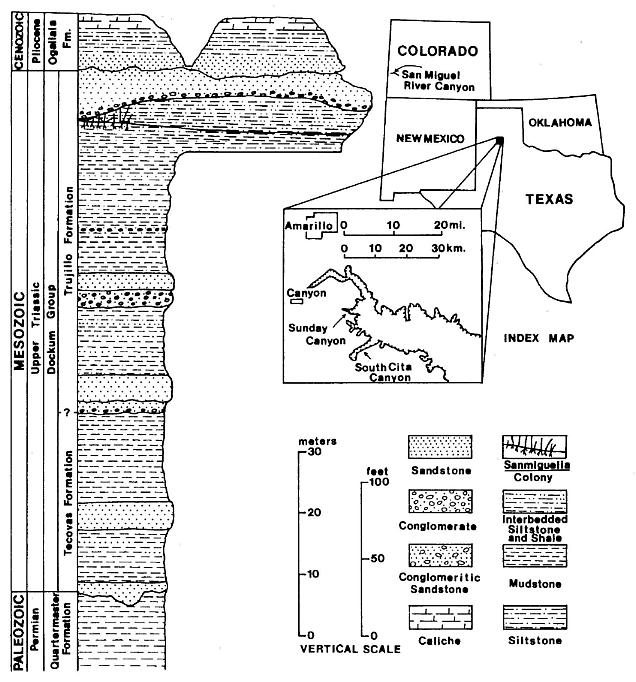
Figure 1. Index map of part of the southwestern United States and a stratigraphic section of the rocks exposed in the Sunday Canyon branch of Palo Duro Canyon, Texas. The approximate position of the locality containing Sanmiguelia leaves and reproductive remains in Sunday Canyon is indicated on the section (modified after Ash, 1976, Text-fig. 1).
forming distinct cross veins. Leaf surface strongly marked by numerous longitudinal plications running parallel to veins from base to apex. Quaternary and tertiary veins fusing in the leaf apex to form larger veins as vein density increases. Cross veins interconnect reformed "secondaries" (based on width only) in apex as smaller veins disappear. Primary veins broad and massive, restricted to base of leaf and sheathing leaf base. More persis- tent primaries as well as secondaries frequently occupying folds in plications. Tertiary and quaternary veins emerge from and anastomose with vein of origin to form elongate narrow loops, unite with adjacent vein immediately after emerging or after forming a long parallel vein, or cross one or more veins before uniting with a vein of equal or lower rank. Small leaves tend to have fewer vein orders, with very small apical leaves or bracts having one large central vein and a pair of small marginal veins.
Sanmiguelia lewisii Brown emend. sp.
LECTOTYPE:
USNM 167538.
HYPOTYPE: BYU 1512, 1584,
1585.
REFERENCES:
1956 Sanmiguelia lewisii Brown, U.S. Geol.
Surv. Prof. Pap. 274H, p. 205-209, Pl.32, figs. 1-2; Pl. 33, fig. 2.
1961 Sanmiguelia
lewisii, Andrews, p. 172, fig. 6-1.
1962 Paloreodoxites lewisii
(Brown) Bock, Geol. Cent. Res. Series, V. 2, p. 283-285, fig. 504.
1963 Sanmiguelia lewisii,
Arnold, Col. J. Ind. Bot. Soc.,
V. 42A, p. 4-9, Pl. 1, fig. 1.
1964 Sanmiguelia lewisii,
Becker, Gard. Journ. p. 231-233. 1969 Paloreodoxites lewisii (Brown) Bock, Geol. Cent.
Res. Series, V. 3, p. 242-254, figs. 402, 403, 407.
1972 Sanmiguelia
lewisii, Becker, Palaeontogr., V. 138B, p. 181-185, Pl. 38, figs. 3-4.
1976 Sanmiguelia lewisii,
Ash, J. Paleont., V. 50, p. 799- 804, text-fig. 2.
1976 Sanmiguelia
lewisii, Hughes, Palaeobiology of Angiosperm Origins, Cambridge Univ. Press, p.
175-179, fig. 13.1, Table 13.1.
1977 Sanmiguelia lewisii,
Tidwell et al., Palaeontogr. V. 163B, p. 143-151, Pl. 1, figs. 1-2, 4; Pl. 2, figs. 2-6;
Pl. 3, fig. 4; text-fig. 4.
NEW MATERIAL: PP34337-PP34343, PP34345-PP34358, PP34410-PP34416: stems, roots, rhizomes; PP34344, PP34359-PP34409: leaves.
NUMBER OF SPECIMENS EXAMINED: 80 (leaves and stems in organic connection above paleosol counted as one specimen).
ILLUSTRATIONS: Pl. 1, figs. a-h; Pl. 2, figs. a-k; Pl. 3, figs. g-i; Pl. 4, fig. i; Pl. 7, figs. m-n; Pl. 8, figs. a, d-i; figures 2-4.
EMENDED DIAGNOSIS: As for the genus. Erect plant, at least >5 cm in height. Each primary axis or stem usually with its own downwardly dividing root stock. Plant typically in vegetative clusters of two or three stems spaced 25 cm to 45 cm apart and connected by subsoil rhizomes. Some stems of a vegetative colony lacking a root system, arising directly from a rhizome. Occasionally two stems originated from the same root stock, and additional stems arose to replace those broken off near their bases. Satellite plants, spaced 85 cm to 175 cm from vegetative clusters, connected to an underground rhizome system.
Leaves spirally arranged around a stem 3-4 cm in diameter. Stems consisting of a large central pith 10-18 mm in diameter surrounded by a woody cylinder, which developed in the lower part of the stem outside a ring of primary vascular bundles, and is broadest at the base of the plant. Lower and upper leaves of mature plant reached 30 cm in length by 19 cm in width with 10-12 plications. Middle leaves reached 48-60 cm in length by 28-31 cm in width with 24 plications. Leaves smaller near base of plant, decreasing in length down to 5 cm with 10 plications. Sheathing leaf bases up to 7 cm long. Leaves of secondary branches much smaller, decreasing to cataphylls 1 cm or less in length at ends of branch. Secondary branches arising between sheathing leaf bases and stem, but not demonstrably in the axils of leaves. Secondary branches capable of assuming upright growth and contin- uation of primary axis if main stem broken.
Medium to large leaves with four orders of venation, parallel to the plications. Vein orders defined by width: Primary veins 1.0 mm-2.5 mm in width, forming 4 mm-11 mm wide longitudinal strips of lamina containing recognizable anastomosing and bifurcating vascular bundles. Primary veins separated mainly by tertiary and quaternary veins, with secondary veins arising from division of primaries. Secondary veins 0.4 mm-0.99 mm in width. Tertiary veins 0.1 mm-0.39 mm in width. Quaternary veins less than 0.1 mm in width down to single strand of tracheids. Primary, secondary, and tertiary veins composed mainly of scalariform tracheids; tertiary and quaternary veins composed of scalariform or reticulate tracheids; quaternary veins sometimes composed of single or double strands of annular-helical tracheids. Larger veins sometimes with associated resin cells and elongate bodies of resin. Cross veins joined all orders of parallel veins, occurred within bundles of larger veins, and formed loops and interconnections that resemble an imperfect reticulum. A small vein of tertiary rank followed margins of leaf, and was joined by crossing tertiary and quaternary veins. Stomata abundant and possibly actinocytic, tending to be oriented perpendicular to parallel veins. Stomata tending to be oriented parallel to veins between some closely-spaced veins. Epidermis thin, moderately covered with single hairs and dendroid trichomes.
DESCRIPTION:
Sanmiguelia lewisii evidently exhibited vegetative reproduction in which
clusters of vertical stems arose from underground rhizomes (figure 2). Branching root
systems extended downwards at least 8 cm below the top of the paleosol, with two or three
major divisions of the primary root occurring within the top 4 cm of the paleosol. Traces
of small roots ocurred as far down as 15-20 cm below the top of the paleosol, but
due to their variable paths through the friable clayey siltstone, organic connection with
the roots of Sanmiguelia could not be verified. Some siderite petrifactions of
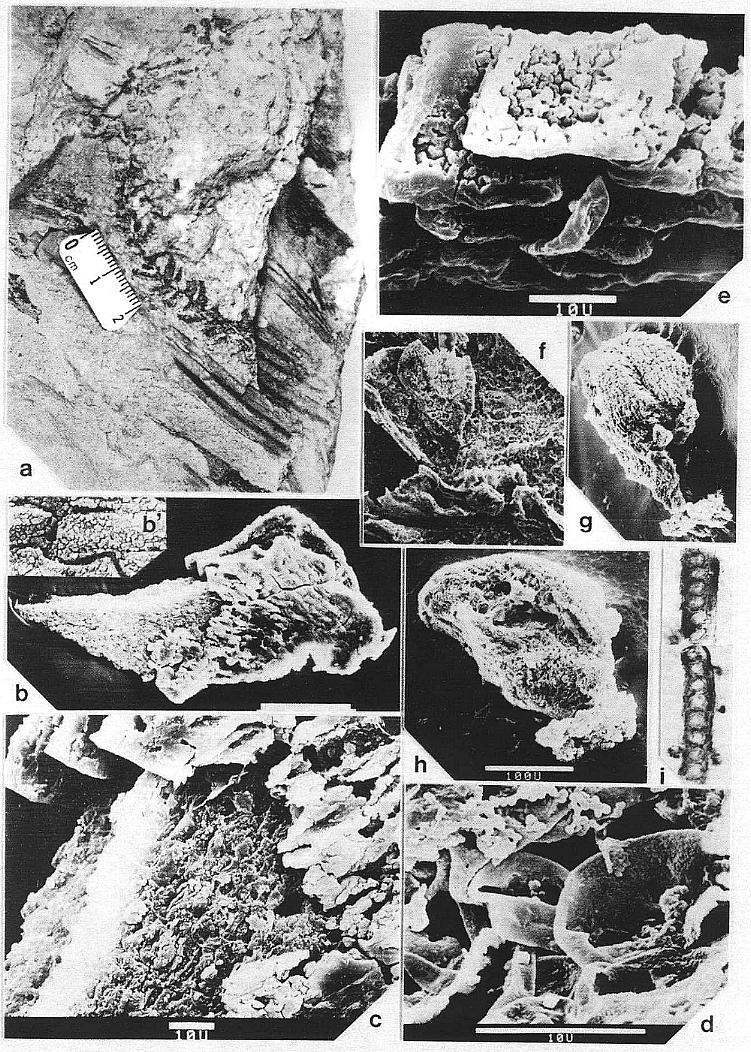
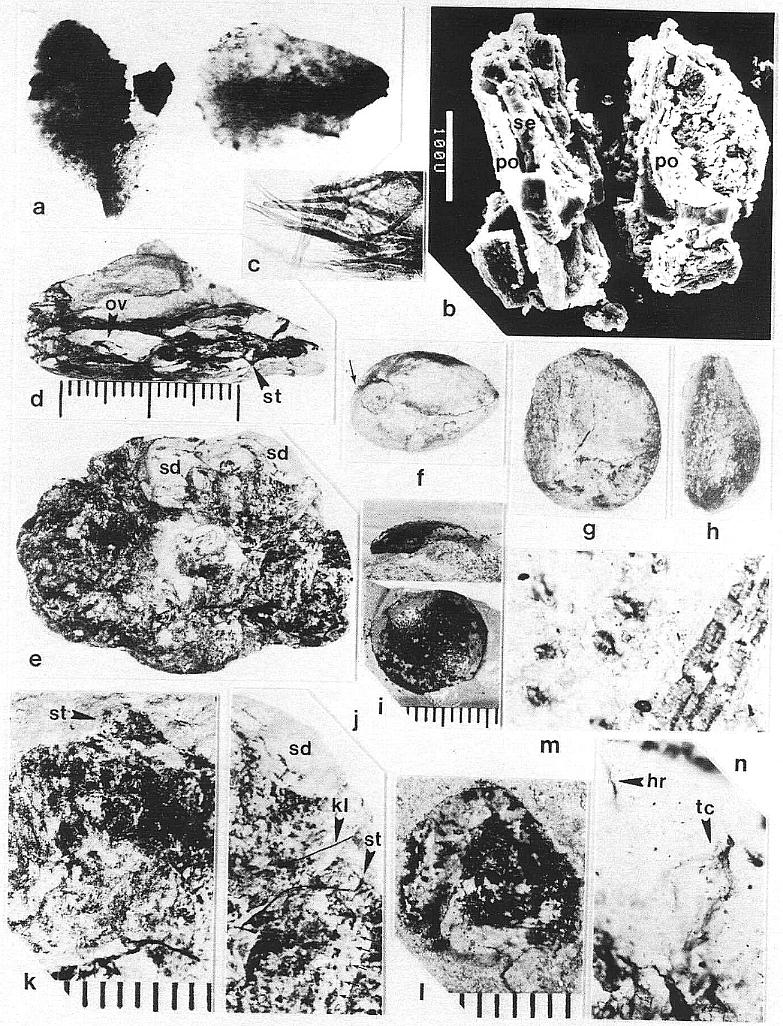
primary roots (Pl. 8, fig. a) yielded excellent preservation, showing a subrounded
(terete) woody stele, possible cambium, scattered vessels, and tracheids possessing
crowded simple pits and sca1ariform end plates (Pl. 2, figs. c-e).
Although the differences in preservation between the Colorado and Texas localities precluded a direct comparison of leaf shape and size along the stems (most, but not all, of the leaves along the stems at Sunday Canyon were missing, and the stems had collapsed around sediment-filled pith casts), leaves were found in the sha1ey sediments on top of the paleosol. Some of those were attached to fallen stems (Pl. 1, figs. a and b; Pl. 5, figs. a, b, and f). All the leaves, fallen or attached to upright stems, fit the criteria of Sanmiguelia based on descriptions and illustrations of leaf impressions (cf. Tidwell et a1., 1977). The added similarity of growth habit and size for the entire plant permits the identification of the Sunday Canyon fossils as S. lewisii.
The form, size, and disposition of the leaves on the main axis are adequately described by Tidwell et a1. (1977), and need not be repeated here. The venation, epidermis, and stomata, however, are preserved in the new material, and are described below:
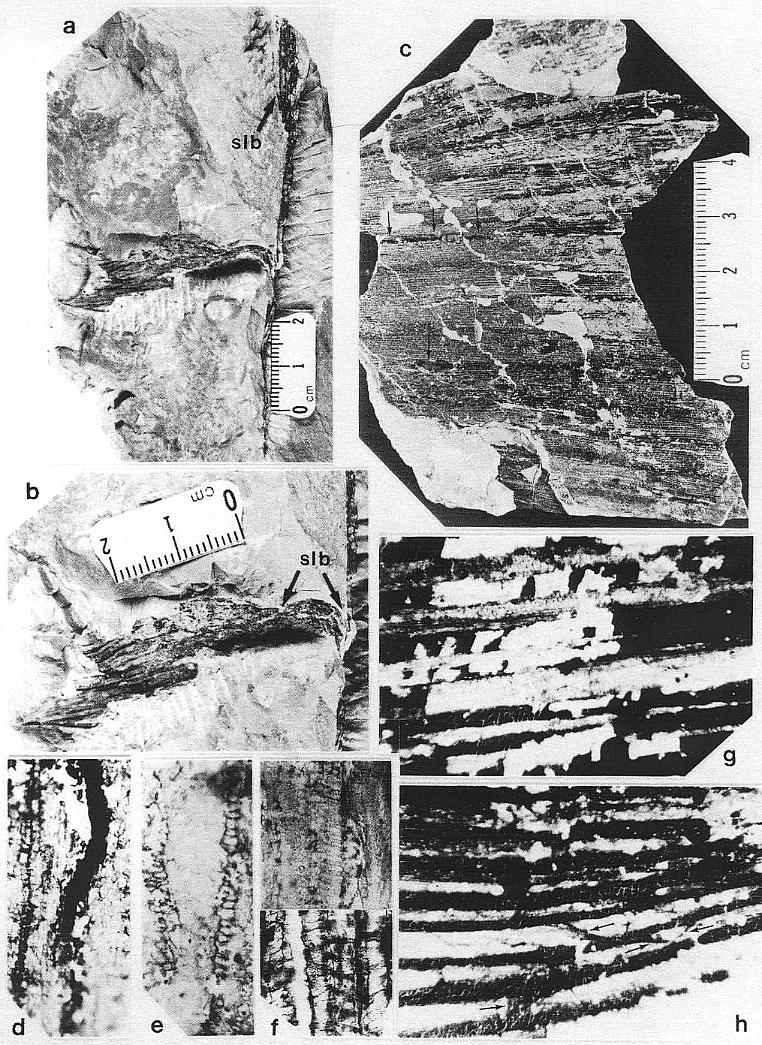
The leaves of Sanmiguelia lewisii possess four orders of parallel veins. These orders are based on width (see diagnosis), since veins of higher rank diverge at low angles from veins of lower rank, and arise from them either laterally or by unequal division or dichotomy (Pl. 1, fig. h; figures 3g and 3h). All but the smallest veins were observed to divide, and most types of veins, with the exception of primaries, were observed to anastomose with veins of equal or lower rank. Some veins were observed to end blindly, although this was attributed to damage during preservation or preparation. Primary veins are restricted to the base of the leaf (Pl. 2, fig. h), where they arise from an elongate sheathing leaf base containing numerous closely-spaced wide veins. As the primary veins divide upwards in the basal part of the leaf blade, they form bundles of smaller primaries separated mostly by tertiary and quaternary veins. Secondary veins are the direct continuation of primary veins as their thickness is reduced through division. Secondary veins rarely form cross veins, and either arise through the upwards division of primaries (Pl. 1, figs. g and h), or reform in the leaf apex through anastomosis (Pl. 3, fig. f; figure 3i).
Tertiary and quaternary veins comprise the bulk of the veins in any portion of the leaf, except its base. Whereas primary and secondary veins tend to occupy the folds in the p1ications, the tertiary and quaternary veins span across the p1ications (Pl. 1, fig. c). Tertiary veins occasionally form cross veins (Pl. 2, fig. k), but usually parallel other veins until they drift toward a vein and fuse with it (Pl. 2, fig. j). Such fusion is usually only temporary, since the resulting vein eventually divides (except in the leaf apex). Quaternary veins, by contrast, are more versatile, since they form parallel veins, vein loops, and cross veins (Pl. 2, fig. j; figures 3g and 3h). Quaternary veins are also the most diverse in terms of composition of their vas- cular tissue, which consists of annular-helical (Pl. 1, fig. e), scalariform (Pl. 2, figs. g and j; Pl. 3, fig. h), and, reticulate (Pl. 7, fig. m) tracheids.
Whereas leaf impressions from Colorado do not preserve any evidence for cross veins, transfer preparations of leaves from Texas show abundant evidence for them (Pl. 1, fig. h; Pl. 2, figs. g and j; Pl. 3, figs. hand i; figures. 3g, 3h, and 3i). Cross veins are typically less than O.1 mm wide, and most are similar in width to quaternary veins. Occasionally, quaternary veins can turn into cross veins as they deviate from their previously parallel course and join an adjacent vein (Pl. 2, fig. j). Cross veins either join adjacent veins directly (Pl. 1, fig. h, at arrows; Pl. 3, fig. h; figures 3g and 3h), or cross one or more veins before rejoining a vein (Pl. 3, fig. i; figure 3g). Veins that naturally cross others were distinguishable from the occasional vein that was displaced or distorted during preservation, because both ends were observed to join other veins. Cross veins even form loops, crossing one vein more than once (Pl. 3, fig. i; figure 3g). Cross veins can be found within the lamina of the leaf between plications, between closely spaced veins in the folds of pllcations, and between the crowded veins in the leaf apex. Cross veins are typically composed of scalariform tracheids (Pl. 3, fig. h), but like quaternary veins, can contain reticulate or annular-helical tracheids.
Measuring the density and percentage of each vein type gives an indication of changing vein morphology from the base to the apex of a leaf (summarized in Table I):
Two leaf fragments measuring 17 mm and 13 mm wide from near the base of a large leaf have vein densities of 1.76/mm and 1.822/mm, respectively. The narrower leaf fragment has one primary (3%), six secondaries (20%), 10 tertiaries (33%), and 13 quaternaries (44%) (Pl. 2, fig. h). The wider leaf fragment has two primaries (6%), three secondaries (10%), 11 tertiaries (35%), and 15 quaternaries (49%).
A large folded leaf fragment measuring 140 mm wide has a vein density of 2.4/mm across the middle 80 mm wide surface exposed on one side of the specimen (Pl. 1, fig. c). The tapering margins and size of this fragment indicate it came from the upper middle of a nearly mature leaf. A significant shift toward smaller veins has occurred, with an absence of primaries, 11 secondaries (6%), 63 tertiaries (32%), and 121 quaternaries (62%).
The percentages of vein types in the leaf apex resembles
that near the base of the leaf, with the exclusion of primaries (Table I). A large portion
of one apex (Pl. 3, fig. f; figure 3i) may represent a leaf that is not fully enlarged,
because it tapers more gradually than the apices of large leaves illustrated by Tidwell et
al. (1977). The lower part of this fragment is 37
mm wide, and has a vein density of 2.2/mm. The density increases in the 10 mm wide leaf
tip to 3.4/mm. Primary veins are absent, while secondaries increase from two (2%) to nine
(27%) going into the tip. Tertiaries decrease from 36 (44%) to 11 (32%), and quaternaries
decrease from 45 (54%) to 14 (41%).
By contrast to the above leaf fragments, which could all have come from leaves of similar size, another leaf fragment (Pl. 3, fig. i) shows a significant increase in quaternary veins. This 30 mm wide fragment may represent the widest portion of the lamina in the upper part of a fully expanded mature leaf. Vein density is 2.9/mm. There are only two secondaries (2%), 10 tertiaries (12%), and 75 quaternaries (86%), many of which nearly reach the lower width limit (0.1 mm) for tertiary veins (figures 3g and 3h).
A 22 mm wide fragment from the lower half of a smaller leaf demonstrates how quaternary veins could have increased through the lateral division of tertiary veins in a leaf expanding by means of an intercalary meristem. Vein density is 2.1/mm, which is intermediate between values for the base and middle of a leaf. There are only four secondaries (8%), 31 tertiaries (66%), and 12 quaternaries (26%).
| Portion of Leaf: Vein Density: Quaternaries: Tertiaries: Secondaries: Primaries: |
BASE 1.76-1.82 44-49% 33-35% 20-10% 3-6% |
LOWER 2.1* 26% 66% 8% -0- |
MIDDLE 2.4 62% 32% 6% -0- |
UPPER 2.2-2.9 54-86% 44-12% 2% -0- |
TIP 3.4 41% 32% 27% -0- |
* Possibly from a smaller less mature leaf.
Some of the larger veins, particularly those along the folds of plications, occasionally have elongate narrow bodies of resin associated with them (Pl. 1, figs. c at arrows and d; Pl. 3, fig. i at arrows). High magnification shows these bodies to be comp- posed of small beads of resin (1.5-20 um in diam.), which either parallel or overlie a vein between the cuticles, and follow the veins for distances up to 2.5 cm (Pl. 1, fig. d; Pl. 2, fig. i).
Leaf cuticles are thin, and cell outlines are
difficult to observe, perhaps due to the superposition of upper and lower cuticles (Pl. 3,
fig. g; Pl. 7, fig. m). Most epidermal cells appear to be very small (on the order of 8-14
um long). Between closely-spaced veins, particularly in the lower part of the leaf, cells
are longer and occur in files parallel to veins (Pl. 1,
fig. d; Pl. 3, fig. h). Epidermal cell shape is more isodiametric and variable, however,
over most of the cuticle, particu1ar1y in the upper expanded parts
of the leaf (Pl. 2, fig. g; Pl. 3, fig. g; Pl. 7, fig. m). Stomata are variably preserved,
but usually stand out as pairs of dark oval guard cells surrounded by probable subsidary
cells of varying wall thickness. The subsidary cells normally have
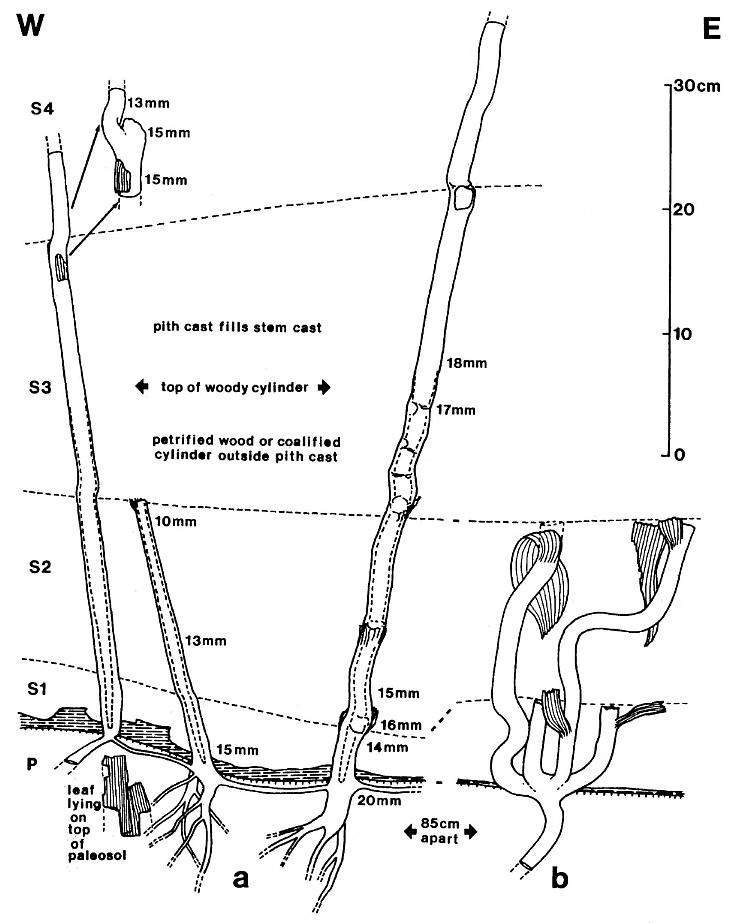
Figure 2. Sanmiguelia lewisii Brown, preserved at the Sunday Canyon locality as sediment-filled pith casts, which terminate at various levels (Sl-S4) within shallow lacustrine and overbank deposits, and which are rooted in a paleosol (P) underlying the entombing silt-dominated sediments. Note small plicate leaves and leaf bases attached to some axes, dark gray shale overlying paleosol (shaded pattern), subsoil rhizomes interconnecting the axes, and branching roots within the paleosol. Changing widths of stems are given in mm and the distribution of secondary xylem is indicated by a basally increasing gap between stem and pith cast diameters.
thicker walls than epidermal cells, making them apparent even when epidermal cell outlines are not obvious. Frequently, a subsidary cell at each end of the guard cells has dark walls, giving the stomatal apparatus a dumbbell shape (Pl. 2, fig. g; Pl. 3, fig. g). The tracing of subsidary cell outlines, with some inferred boundaries, suggests that a ring of six to eight cells surround the guard cells (figures 3j and 3k). The stomata appear to be of the actinocytic type, but would be of the anomocytic type if surrounding cells were interpreted as indistinguishable from those of the epidermis (Dickison, 1975). The long axes of the stomata are typically oriented perpendicular to the parallel veins (Pl. 2, fig. g; Pl. 3, fig. g; Pl. 7, fig. m). The high apparent density of stomata may be due in part to the superposition of upper and lower cuticles, although some cuticles of apparent single thickness also had closely-spaced stomata. Stomata appear to be present on both sides of the leaf (amphistomatic).
In transfer preparations of leaf cuticles where the rock
matrix was dissolved or disaggregated, epidermal trichomes were found to form a moderate
cover of very small (22-35 um long) single and dendroid hairs. In places where the cuticle
was either missing or very thin, trichomes were observed and photographed (Pl. 7, fig. n).
Most of the trichomes appear to be simple, possibly unicellular hairs, while cell walls
within them. The dendroid hairs have a short 16 um long stalk, from the top of which
radiates a cluster of five, 8-19 um long hairs (figure 31). The apical hairs are
constricted where there seem to be cell walls. The apical hairs appear to join the stalk
in pairs, a condition found in dendroid trichomes, but not in stellate ones (Esau, 1960).
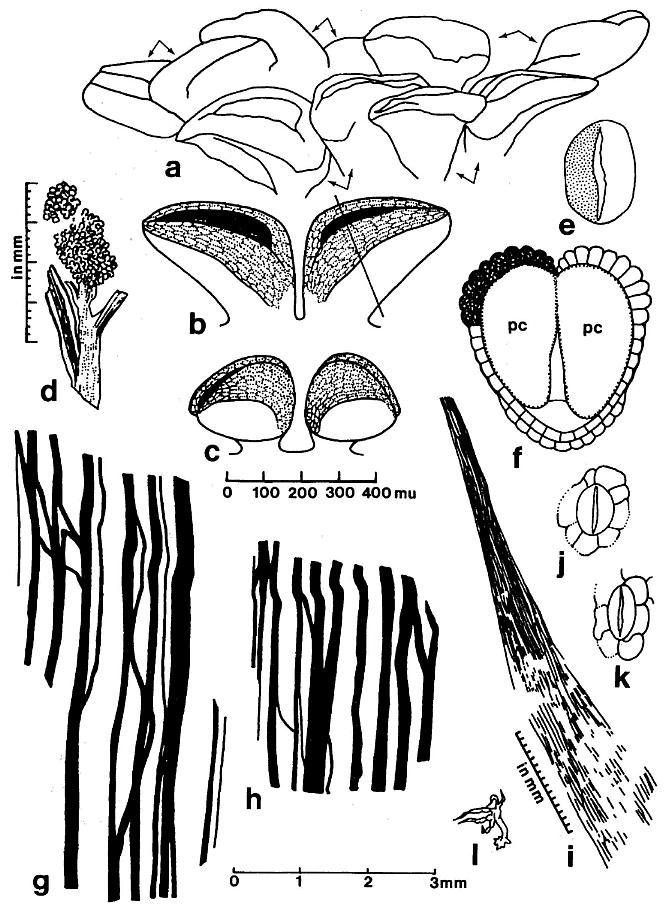
Figure 3. a-1. Line drawings and restorations of Sanmiguelia lewisii pollen-bearing organs and leaves; four different scales given for a-c, d, g-h, and i, or below. -a. Synangispadixis tidwellii microsporophy11s, showing paired arrangement. -b. Restoration of pair of mature biloculate microsporophy11s, showing band of enlarged endothecia1-1ike cells on either side of longitudinal suture. -c. Restoration of pair of immature biloculate microsporophy11s. -d. Distal portion of a S. lewisii secondary branch ending in one synangia-1ike microsporophy11-bearing organ of S. tidwellii (Pl. 5, fig. d). -e. Pollen grain of S. tidwellii, showing granular intrastructure on one side,psi1ate sculpture on other; x 1167. -f. Cross section of microsporopy11 at line in figure 3b, showing endothecia1 cell layer,proximal epidermis, and septum dividing two pollen chambers (pc). -g and h. Sanmiguelia lewisii, camera 1ucida enlargement of leaf venation (Pl. 3, fig. I); note veins drawn slightly wider and not to scale. -i. S. lewisii, camera 1ucida drawing of anastomosing vein bundle in leaf apex (Pl. 3, fig. f). -j. S. lewisii, camera 1ucida drawing of actinocytic stomata (Pl. 7, fig. m); x 204. -k. S. lewisii, camera 1ucida drawing of actinocytic stomata (Pl. 3, fig. g); x 230. -l. S. lewisii, camera 1ucida drawing of dendroid trichome (Pl. 7, fig. n); x 105.
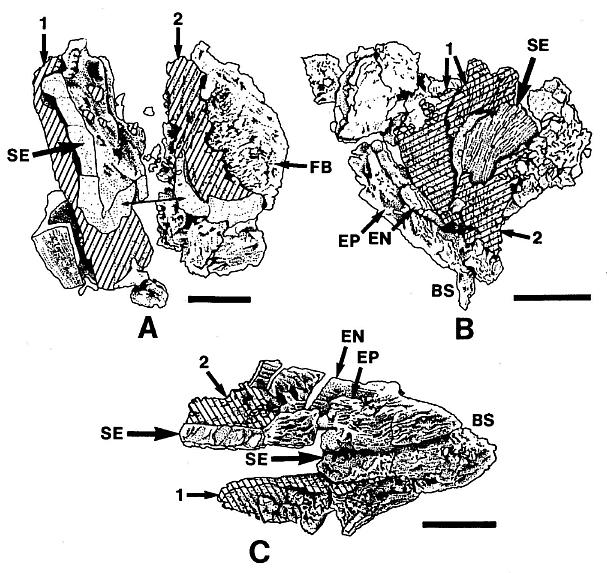
Figure 4. a-c. Line drawings from SEMGs of microsporophy11s to Synangispadixis tidwellii Cornet sp. nov. PP34322. "Mummified" microsporophy11s broken open to reveal two pollen masses (cross hatched) of different size (1 larger than 2), separated by a septum (SE) of variable development, thick-walled "fiber" cells (FB) adjacent to the suture, probable epidermal (EP) and endothecia1 (EN) cell layers near base (BS), and outlines of individual pollen grains; photograph of specimen a. in Pl. 7, fig. b; bar scales 100 um long. Note outer pieces of wall removed to reveal internal structure.
DISCUSSION: There is no true petiole, only a leaf base that narrows to meet and wrap around the stem. Within the lower part of the leaf blade primary veins are typically composed of bundles of smaller veins. Sheathing leaf bases preserved along the vertical axes show closely-spaced veins that are as wide or wider than the primaries that pass into the leaf blade. As the stem enlarged through secondary growth, the sheathing base eventually split down the opposite side of the stem from which the leaf blade diverged (Pl. 2, fig. a). Only small leaves have been found along the lower part of the stem where secondary growth and stem width were the greatest (cf. Tidwell et a1., 1977; figure 2).
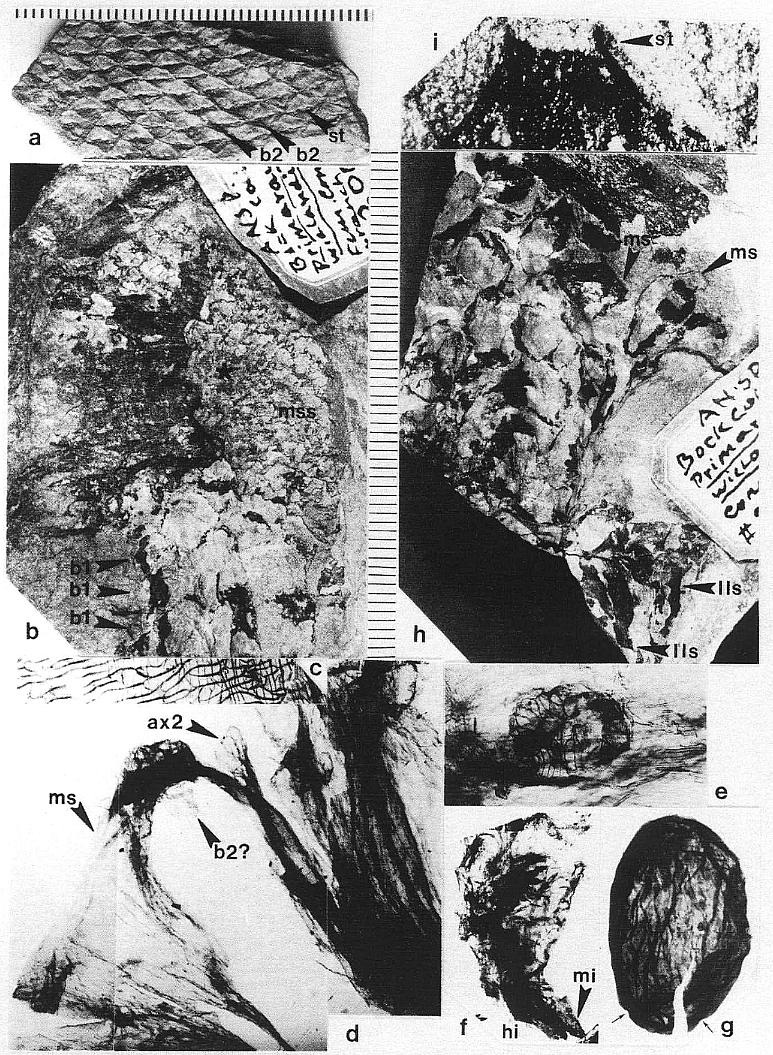
Vascular traces were followed in thin section as they passed through a 1.1 cm thick "cylinder" of wood lying outside a vertically striate pith cast (Pl. 2, fig. a). Pith cast diameter varies along the stem (figure 2), and its striate outer surface was formed by elongate endarch protoxy1em bundles, which are sometimes preserved along with the secondary xylem (Pl. 8, fig. e). A broad zone of smaller tracheary elements separates the protoxy1em and secondary xylem (Pl. 8, fig. e). The leaf traces are circular in cross section and pass out through very tall wide gaps in the xylem that resemble giant parenchymatous mu1tiseriate rays (Pl. 8, fig. i). Uniseriate rays characterize the vesse1less wood between the wood gaps (Pl. 8, figs. f-h). The wood contains tracheids with he1ica1-sca1ariform (Pl. 1, fig. f) and circular bordered pitting (Pl. 4, fig. i). Several wood gaps occur in parallel, and the circular traces divide within them as they course outward, becoming stacked vertically. The traces arise from a ring of many protoxy1em bundles surrounding the pith. They were observed to diverge upwards through the wood gaps, and apparently passed through a 3-4 mm thick cortex before entering a leaf base (based on measured diameters of stem, wood, and pith).
In the upper part of vertical stems the pith cast lacks evidence of secondary growth and shows very wide and elongate vascular gaps spirally arranged around the pith (Pl. 8, fig. d). The number of grooves and ridges along the pith cast is fewer than along the lower part of the stem, and the grooves intersect the tapering ends of the gaps as though protoxy1em bundles diverged away from the pith as they entered a gap, then returned to the perimeter of the pith above the gap. Whereas leaf traces occupy multiple parallel gaps of much narrower width in the secondary xylem (Pl. 8, fig. i), distinctive leaf gaps may have arisen distally above the level where secondary growth stopped. Although protoxy1em bundles appear to be restricted to the perimeter of the pith where secondary xylem occurs, they may have diverged away from the pith higher on the stem in areas of leaf trace origin. If leaf gaps widen to the extent of touching one another in the uppermost part of the stem, the distribution of primary xylem bundles might resemble that found in a Ranuncu1us or monocot stem. A vascular pattern of that type may be preserved in the main axis of the pollen-bearing inflorescence associated with Sanmiguelia (Pl. 3, fig. a).
Although the secondary xylem of aerial stems lacks vessels, the roots appear to possess them. A subcircular woody core (distorted by compression?) in a petrified root (Pl. 8, fig. a) contains tracheids of increasing size outwards (Pl. 2, fig. c), but the tracheids, formed by a distinctive cambium, also become more and more unequal in size outwards, resulting in a two-three fold difference in diameters between the smallest and largest elements. The larger elements are interpreted as vessels, because they form separate vertical files, and their end plates appear to be simple perforations (Pl. 2, fig. d, arrow), while the end plates of the smaller tracheids are scalariform (Pl. 2, fig. e). By contrast, a petrified rhizome with evidence of sheathing cataphylls contains a small central diarch protostele with no apparent secondary growth, surrounded by a large cortex. It is unusual for a plant with rhizomes lacking secondary growth to produce woody stems and roots, but it is equally unusual for a plant with woody stems to have non-deciduous sheathing parallel-veined leaves and leaf gaps that appear to have changed significantly in morphology from stem base to apex. More information on stem, rhizome, and root anatomy will be presented elsewhere.
The most striking feature of Sanmiguelia leaves is the number of small veins that leave no impression if they are not preserved. Although the primary and secondary veins tend to be well organized and follow the folds of plications in the blade, the finer venation is generally disorganized, particularly at the quaternary level. The width of intercostal areas (areas between secondary veins) varies along the length of the leaf, and extends across plications as secondary veins bifurcate and divide in the upper part of the leaf. The irregularly ramifying courses of some tertiary and many quaternary veins, as well as the poor differentiation of all vein orders (they are distinguished mainly by width rather than position or pattern of origin) gives these leaves a very primitive appearance, despite their resemblance to the leaves of extant monocots (Tidwell et al., 1977). The disorganized venation of Sanmiguelia leaves is more comparable to that of Zone I angiosperm leaves from the Potomac Group (Doyle and Hickey, 1976).
Very large single-bladed undivided leaves are very rare in the fossil record, and are rarely known outside the angiosperms. The ontogeny of a large simple leaf must involve more than a marginal meristem if the veins come together and fuse in the leaf apex rather than run parallel and end blindly at the distal margin. The venation of Sanmiguelia leaves indicates that they were narrower before unfolding from the stem, and that they enlarged in width through intercalary expansion to reach their mature size and shape. An apical meristem can account for the length, but a marginal meristem cannot account for the post-elongation width of Sanmiguelia leaves, because of the bifurcation and anastomosis of tertiary and quaternary veins between parallel veins, well inside the margins of the leaf, and because some cross veins wander considerable distances, crossing other veins as if they formed late in leaf development. The frequent absence of preserved leaf tips (Tidwell et al., 1977; Pl. 3, fig. f), even for the smallest leaves (Pl. 5, fig. d), suggests that the apical meristem ceased its activity before the leaf reached full size, making the leaf tip susceptible to injury and damage.
Apical venation resembles leaf-base venation (Table I), and primary and secondary veins are typically composed of bundles of smaller veins (Pl. 2, fig. h), suggesting that mesophyll initials were intercalated between bundles of procambial initials. As the leaf lamina widened during its initial stages of growth, smaller veins emerged by the development of mesophyll initials between differentiating closely-spaced procambial bundles. If mesophyll initials did not proliferate, a wide vein formed like that found in the clasping leaf base and in small apical leaves on determinate secondary branches (Pl. 5, fig. d). As mesophyll tissue differentiated between developing vascular bundles, the direction of primary growth was lateral, rather than apical. Lateral growth resulted in the orientation of guard cell pairs parallel to the direction of growth, or perpendicular to the veins (Pl. 2, fig. g; Pl. 7, fig. m). The orientation of guard cells is direct evidence for intercalary growth and plate meristems between parallel veins in Sanmiguelia leaves. Maturation appears to have progressed basipetally from the narrow apex to the widest portion of the leaf, then decreased towards the leaf base. Only between closely spaced veins that experienced minimal lateral growth (mainly near or at the base of the leaf) are stomata and epidermal cells oriented predominantly parallel to the veins.
POLLEN-BEARING REPRODUCTIVE STRUCTURES OF SANMIGUELIA
Synangispadixis Cornet gen. nov.
TYPE SPECIES: Synangispadixis tidwellii Cornet sp. nov.
DIAGNOSIS: Reproductive axis without apparent bracts or leaves, tapering apically, bearing hundreds of helically arranged secondary axes; main axis wide at base, long, flexible. Secondary axes covered with hundreds of sessile, biloculate, double-walled microsporophylls, each with a constricted base. Micro- sporophylls arranged in opposite pairs. Microsporophyll pairs arranged in a tight spiral around immature secondary axes. Secondary axes along basal part of main axis elongate, wide ("fleshy" or expanded), with loosely-arranged dehisced microsporophylls. Secondary axes higher on main axis progressively shorter, narrower, with undehisced microsporophylls containing immature pollen. At apex secondary axes bear smaller immature microsporophylls. Microsporophylls elliptical to elongate, having an adaxial outer wall of thickened endothecial or "fiber" cells, an epidermal cuticle along constricted microsporophyll base, and a longitudinal adaxial slit over a septum separating two pollen masses. Each pollen mass surrounded by the remnants of a tapetum. Septum largely disappears at maturity, producing one united pollen chamber containing hundreds of small, elliptical, psilate tectate-granular monosulcate pollen.
DERIVATION: From syn - Greek, meaning together, with, united; angio - Greek, meaning vessel or container of any kind, capsule, seedcase (or sporecase) of plants; spadix - Greek, meaning a spike of flowers on a fleshy axis, whether perfect or imperfect, naked or withperianth; also synangia - plant structures with united microsporangia.
DISCUSSION: This genus is established for the large male reproductive axes associated with Sanmiguelia, and which probably terminated the main axis of Sanmiguelia lewisii. The secondary "fleshy" branches covered with crowded sessile microsporophylls closely resemble synangia borne along the spadix of some aroids. The condensation of microsporophylls on specialized branches is interpreted as a type of synangia, even though the microsporophylls are not joined to each other. The enlargement of the secondary axes at the time of pollen dehiscence is comparable to the enlargement of aroid spadices during anthesis.
Pollen-bearing structures identical to the secondary branches of Synangispadixis terminate some secondary vegetative branches of Sanmiguelia. They are borne individually or in clusters of up to three, suggesting that the secondary branches of Synangispadixis are specialized organs (i.e. synangia) and not just branches bearing microsporophylls. The organic connection of these organs to Sanmiguelia supports the interpretation that Synangispadixis belongs to Sanmiguelia, but since Synangispadixis was not found in organic connection with Sanmiguelia and it represents a distinctive reproductive structure, it is given its own name. Should Synangispadixis and Sanmiguelia be found in organic connection, the name taking priority will depend on the systematic treatment of the leaves.
Synangispadixis tidwellii Cornet sp. nov.
HOLOTYPE:
PP34322. HYPOTYPE: PP34323.
DIAGNOSIS: As for the genus.
REFERENCES: 'unknown cone' adjacent to 'Paloreodoxites lewisii', Bock (1969: 245, fig. 405, photograph & line drawing).
OTHER MATERIAL: PP34326-PP34327.
RELATED MATERIAL: PP34324-PP34325, PP34328.
NUMBER OF SPECIMENS EXAMINED: 7.
ILLUSTRATIONS: Pl. 3, figs. a-e; Pl. 4, figs. a-h; Pl. 5, figs. a-e, g; Pl. 7, figs. a-b; figure 8b.
DERIVATION: After William D. Tidwell, Professor of Botany, Brigham Young University, for his paleobotanical contributions to - our understanding of Sanmiguelia and his courage in publishing - controversial evidence in support of pre-Cretaceous angiosperms.
DESCRIPTION: One virtually complete reproductive axis (Pl. 3, fig. a), the distal portion of a second (Pl. 4, fig. a), and a mature portion of a third (Pl. 3, fig. b) were found intimately associated with Sanmiguelia leaves and stems. These reproductive structures appear to have been borne terminally on the main axis for three reasons: 1) the width and size of their central axis compares with that of the distal portions of Sanmiguelia primary axes; 2) the paniculate branch system lacks bracts or leaves, and is naked for its entire preserved length, which is unlike the leaf-bearing secondary branches of Sanmiguelia that terminate in pollen-bearing organs; and 3) one specimen shows the distal portion of an axis overlying one(?) frayed or ripped Sanmiguelia leaf. The orientation of these specimens is such that they may have been attached (Pl. 4, fig. a; see also figure 8b for an interpretation).
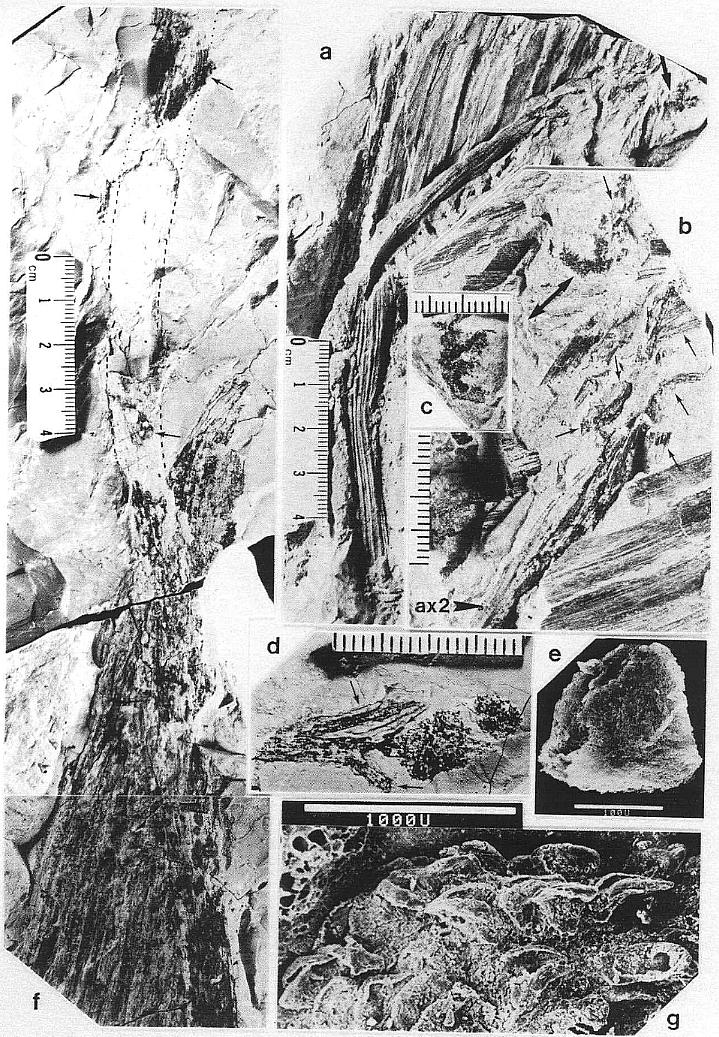
The main axis of the complete reproductive axis (Pl. 3, fig. a) is 24.2 cm long, 14 mm wide at its base, and contains numerous individual vascular strands, which appear to anastomose and bifurcate along the axis. SEMGs of these vascular strands show mostly helical-scalariform tracheids (Pl. 3, fig. a'; also Pl. 1, fig. f). The main axis is naked and branchless for the basal 4 cm, tapers gradually to 8 mm in width where the secondary axes begin, and continues with that width for about 6 cm, or until the secondary axes decrease in length (Pl. 3, fig. a, arrow). The main axis abruptly decreases to 5 mm (at arrow), and thereafter gradually decreases to less than 2.5 mm in width near the apex.
The proximal secondary axes are 18-19 mm in length; this length is maintained for 6 cm along the main axis. Above that point on the main axis the secondaries abruptly decrease in length to 11 mm (Pl. 3, fig. a, arrow). Subsequently, the secondary axes gradually decrease in length apically from 11 mm to about 6 mm. They are spirally attached along the main axis, but due to compression and burial, only the lateral branches can be clearly seen. There are about 40 elongated secondary axes visible along the basal 6 cm, and about 60 secondaries over an 8 cm length of main axis distally. An estimated 80 secondaries surrounded the basal 6 cm of main axis, with over 280 secondaries apically. The elongation and increase in width of the proximal secondaries coincides with maturity, since most of the microsporophylls on those branches have dehisced, while the pollen sacs of microsporophylls on the shorter and narrower distal secondaries (above arrow in Pl. 3; fig. a) are still full.
The secondary axes depart from the main axis at right angles, and, except for the basal 1-3 mm, are entirely covered with sessile biloculate microsporophylls. These secondary axes are interpreted as synangia, even though the microsporophylls are not fused to each other, because very similar structures were found terminating vegetative secondary branches of Sanmiguelia, either individually or in clusters of three or more(?) (Pl. 5, figs. a-d; figure 3d). The similarity of pollen-producing structures suggests that the secondary branches of Synangispadixis are specialized organs that were genetic units, and resulted from the fusion or coalescence of numerous microsporophylls as in a synangium. The vegetative branches of S. lewisii that terminate in reproductive organs have small parallel-veined cataphylls that decrease in size apically. As these cataphylls decrease in size, their venation condenses to a wide central vein flanked by a pair of marginal veins (Pl. 5, fig. d). No cataphylls, bracts, or leaf-like organs were observed on Synangispadixis.
The microsporophylls are bilaterally symmetrical, longer than wide, and basally constricted where they join the secondary axis. Laterally they range in shape from lunate to tear-drop (Pl. 3, fig. c; Pl. 4, fig. f-h; Pl. 5, fig. g; figure 3a-3c). They range in length from 230 to 525 um, and in height from 170 to 351 um. Most are longer than tall (e.g. 330 um by 240 um), while others are taller than long (e.g. 280 um by 351 um). A longitudinal suture runs adaxially across the apex and down the ventral side, and is distally flanked by large rectilinear cells with their short axes typically oriented perpendicular to the suture (Pl. 3, fig. c; Pl. 4, figs. e-g). These cells are much larger adjacent to the suture than along the sides of the microsporophylls, and form a protruding ridge on both sides of the suture (Pl. 3, fig. c; Pl. 4, figs. g-h; Pl. 5, fig. g). The. cells within the ridges appear to contain an internal structure consisting of fiber-like thickenings of the cell walls (Pl. 7, fig. b; figure 4a), while much smaller cells below the protruding ridges contain an internal structure consisting of fused granules (Pl. 4, fig. e). The enlarged cells may be a structural adaptation for the opening of the anther sac. Their external position could be interpreted as indicating an epidermal origin, but their morphology is more comparable to the fibrous endothecial cells of angiosperm anthers, which frequently occupy an external position (an epidermal cell layer disappears early during ontogeny: Eames, 1961). Sometimes remnants of a waxy cuticle are preserved as scabs or beads upon the enlarged cells bordering the suture (Pl. 4, fig. b'). The lower part or base of each microsporophyll appears to be covered by an epidermis (figure 3f), which extends upwards from the axis, forming a wrinkled layer covering an inner endothecial layer (figures 4b-4c, ep; Pl. 3, fig. c; Pl. 4, fig. h). Between the thickened cells forming the ridges bordering the suture and the upper limits of the epidermis, the endothecial layer forms the major part of the wall (figure 3f), and there it commonly shows signs of damage (Pl. 3, fig. c; Pl. 4, fig. h).
The microsporophylls are tightly packed and appear to be spirally arranged along immature secondary axes (Pl. 5, fig. c), but are loosely packed on expanded mature axes (Pl. 3, fig. b; Pl. 5, fig. g). They are borne back to back in pairs on the secondary branches (Pl. 4, fig. f; Pl. 5, fig. g; figures 3a-c), and the sutures of adjacent microsporophylls are opposite one another. The apices of the microsporophylls are frequently pointed (Pl. 4, fig. b; Pl. 5, fig. g; figure 3a), but may also be blunt or rounded (Pl. 4, figs. g, h).
Many microsporophylls contain pollen preserved in two distinct masses, separated by a noticeable septum (Pl. 7, figs. a, b; figures 4a-4c), while others contain only a single mass (Pl. 4, figs. b-c). Of the five specimens broken open and observed under SEM, four possess a septum between two masses of pollen (figures 4a-4c). The septum is massive and amorphous in cross section (Pl. 7, fig. b, se; figures 4a-4c), while outer wall layers commonly show remnants of cellular structure, suggesting that the septum formed from the coalification of cells with thin or weak cell walls. The septum also appears to vary in thickness and extent of development (compare figures 4a-4c). Individual microsporophylls were oxidized and cleared in NaOH in order to determine the number of pollen "sacs". Most of them (presumably the less mature ones) yielded two pollen masses, sometimes with remnants of a septum still attached (Pl. 7, fig. a). A few yielded a single, large bilobed pollen mass with either a long narrow cleft (Pl. 4, figs. b-c) or a broad deep cleft (Pl. 5, fig. e) separating the two lobes. After dehiscence, only one pollen chamber is visible within each microsporophyll (Pl. 4, fig. f; Pl. 5, fig. g).
Pairs of yellow-orange pollen masses isolated from a single microsporophyll frequently are of unequal size (cf. Pl. 7, fig. b; figures 4a-4c). Isolated masses are compressed and range in size from 285 um to 380 urn in length. SEMGs of their surfaces show pollen covered by sporopollenin-coated (acid-resistant) cellular debris and Übish bodies (Pl. 4, figs. c, d). Only the vague outlines of pollen can be seen through this pollen sac wall and tapetal debris (Pl. 4, fig. c; Pl. 5, fig. e). The pollen is small, elliptical, tectate-granular, and monosulcate, but the sulcus is poorly defined and irregular in shape (Pl. 3, figs. e, d; Pl. 4, fig. d; figure 3e). Pollen from mature microsporophylls (e.g. just above arrow in Pl. 3, fig. a) can be partially disaggregated through maceration, while that from immature ones cannot. Mature (oxidized) pollen ranges in length from 21 urn to 29 um (median 24 um), and in width from 11 um to 19 urn (median 15 um). Unoxidized pollen is about half that size (Pl. 4, fig. d). A granular infrastructure can be viewed in transmitted light (Pl. 3, figs. e, d). Internal granae are visible where the tectum either has not completely formed on immature pollen or has been removed (Pl. 4, fig. d). The absence of any internal structure in dispersed pollen of identical overall morphology (from the rock matrix of specimens) suggests that mature Synangispadixis pollen may have lacked any distinctive characteristics which might distinguish it from monosulcate gymnospermous pollen.
DISCUSSION: Synangispadixis tidwellii sp. nov. appears to have matured acropetally over a long period of time (i.e. days or even weeks). Its unisexual condition and long flexible central axis suggest that it was morphologically adapted for wind pollination, but the inflorescence was probably enclosed by a spathe or large protective leaf early in its development, thereby shielding the proximal secondary branches as they reached anthesis (cf. figure 8). Entomophily may have been important during early development, while anemophily may have predominated later when the spathe-like leaf opened up or the inflorescence grew higher than the leaf.
Each microsporophyll had two pollen masses containing an estimated 300 pollen grains each. If each secondary branch bore an estimated 400 microsporophylls (about 200 counted per side), and four branches encircling the main axis matured at one time, about 960,000 pollen grains would have been released at each successive stage of anthesis. A reproductive axis like the holotype (Pl. 3, fig. a) possessed a minimum of 360 secondary branches, and would have released over 345 million pollen grains as it matured. The relative scarcity of simple monosulcate pollen in the sediment or clinging to the cuticles of the leaves (most of the other pollen types in the sediments are found on the leaves) suggests either that Synangispadixis effectively dispersed pollen through the wind to distant localities, or that insects played a role in selectively removing and transporting pollen without much of it falling into the surrounding sediments.
The presence of two distinct pollen masses, which are separated by a septum that disappears with maturity, gives the microsporophylls a morphology and ontogeny like that of angiosperm anthers. The tapetal debris and Ubish bodies surrounding each pollen mass (Pl. 4, figs. c-d), but not present within it (cf. Pl. 3, fig. d), suggest that the tapetum may have been secretory rather than ameboid in function (see Eames, 1961, p. 142). The overall shape, sessile nature, and the extension of a suture most of the way down one (ventral) side of the microsporophyll (Pl. 4, fig. g) give it a primitive carpel-like shape (see figure 10 for comparison). The importance of this resemblance will be discussed under evolutionary significance below.
The occurrence of secondary vegetative branches ending with one or more synangia-like side branches of Synangispadixis suggests that the large reproductive axis was borne at the end of a stem that became progressively more fertile with successive vegetative branches (see figure 8 for a reconstruction). Whether a rhizomatous vegetative colony of three or more closely-spaced stems was monoecious or dioecious is not known for certain, but ovule-bearing and pollen-bearing reproductive branch systems were l found lying very near one another adjacent to vegetative colony A in figure 2.
OVULE-BEARING REPRODUCTIVE STRUCTURES OF SANMIGUELIA
Axelrodia Cornet gen. nov.
TYPE SPECIES: Axelrodia burgeri Cornet sp. nov.
DIAGNOSIS: Long indeterminate primary reproductive axis probably terminating vegetative axis. Main axis unbranched proximally, with at least two orders of branching distally, bearing spirally-arranged, widely-spaced, small parallel-veined clasping bracts on lower half, and long pointed parallel-veined bracts or cataphylls with elongate sheathing bases on upper half. Cataphylls increase in length distally up to first secondary branch, become closely-spaced with overlapping sheathing bases, then decrease in length, and above the last secondary branch are small, spirally-arranged, and borne on a short narrow terminal axis. As many as five secondary axes emerge from between sheathing leaf bases of enlarged cataphylls, one per cataphyll, with each bearing one or more tertiary branches. Secondary branches stiff, easily broken, possibly six-sided with small scale bracts, ending in a large flower-like cluster of reproductive units.
Tertiary branches short, dividing two or three times, each division immediately bearing a long abaxially recurved conduplicate bract, with a single pedicellate cupule-like megasporophyll in its axil. Two dissimilar leaf-like structures join or fuse with base of megasporophyll: Two inner entire bracts covered with long hairs like the megasporophyll, and two outer digitate and glabrous bracts that are divided near their base into five or six long finger-like projections, each with a stiff central vein.
Secondary branches are terminated by numerous (about eighteen) crowded megasporophylls. The megasporophylls are themselves surrounded by a perianth-like structure. This "perianth" consists of perhaps eight or nine parts resembling the bracts of individual megasporophylls borne on tertiary branches, but the hairy bracts and digitate bracts alternate with one another in pseudowhorls. Hairy bracts form the outermost "whorl" of the "perianth", and are themselves surrounded by 3-4 long adaxially recurved conduplicate bracts.
Megasporophylls with tapering base, expanding upwards to a rounded apex, and terminated by a bilobed u-shaped collar encircling a small canal or opening into a hollow (frequently sediment filled) chamber. Megasporophylls carpel-like with apical opening extended on open side of u-shaped stigma-like collar to form a suture. Suture short, only extending slightly down the ventral side of megasporophyll. Two shoulder-like bulges flank the apex of immature megasporophylls, but these are much less prominent in larger megasporophylls. Megasporophylls covered with long multicellular and glandular hairs. Epidermal hairs on bulges possibly more pronounced than hairs on surrounding parts of megasporophyll.
Megasporophylls of varying size. The smallest on tertiary branches possessing fully-formed bracts. Largest megasporophylls at least nine times larger than smallest ones. Initially, the bracts and megasporophylls enlarge together, but the bracts around mature solitary megasporophylls are usually damaged and incomplete. Perianth-like structure around each terminal cluster of megasporophylls apparently persistent.
The ovules or seeds and megasporophylls show significantly different stages of development within a single cluster and on tertiary branches. A pair of small anatropous ovules occurs inside much larger ovary-like chamber of megasporophylls of intermediate size; a pair of developing seeds occurs in megasporophylls of nearly full size; a pair of seeds with well-formed seed coats (resistant to compaction) occurs in megasporophylls of mature size. Ontogenetically, megasporophylls rapidly elongated followed by a slower increase in breadth, while ovules or seeds apparently show exponential growth late in megasporophyll development.
DERIVATION: After Daniel I. Axelrod, Professor Emeritus, University of California, Davis, for his pioneering theories on pre-Cretaceous angiosperm evolution, and insight into the diversification and pre-continental drift migration of angiosperms in the Cretaceous.
Axelrodia burgeri Cornet sp. nov.
HOLOTYPE: PP34316-PP34319 (one specimen in four parts).
HYPOTYPE: PP34320-PP34321.
DIAGNOSIS: As for the genus.
NUMBER OF SPECIMENS EXAMINED: 3.
ILLUSTRATIONS: Pl. 5, fig. f; Pl. 6, figs. a-c, g-l; Pl. 7, figs. c-e, j-k; Pl. 8, figs. b-c.
DERIVATION: After William C. Burger, Field Museum of Natural History, Chicago, for his progressive revision of the monocot theory of angiosperm evolution, concept of floral evolution from the condensation of simple reproductive units, and recognition that monocots possess characteristics that may be primitive for angiosperms as a whole.
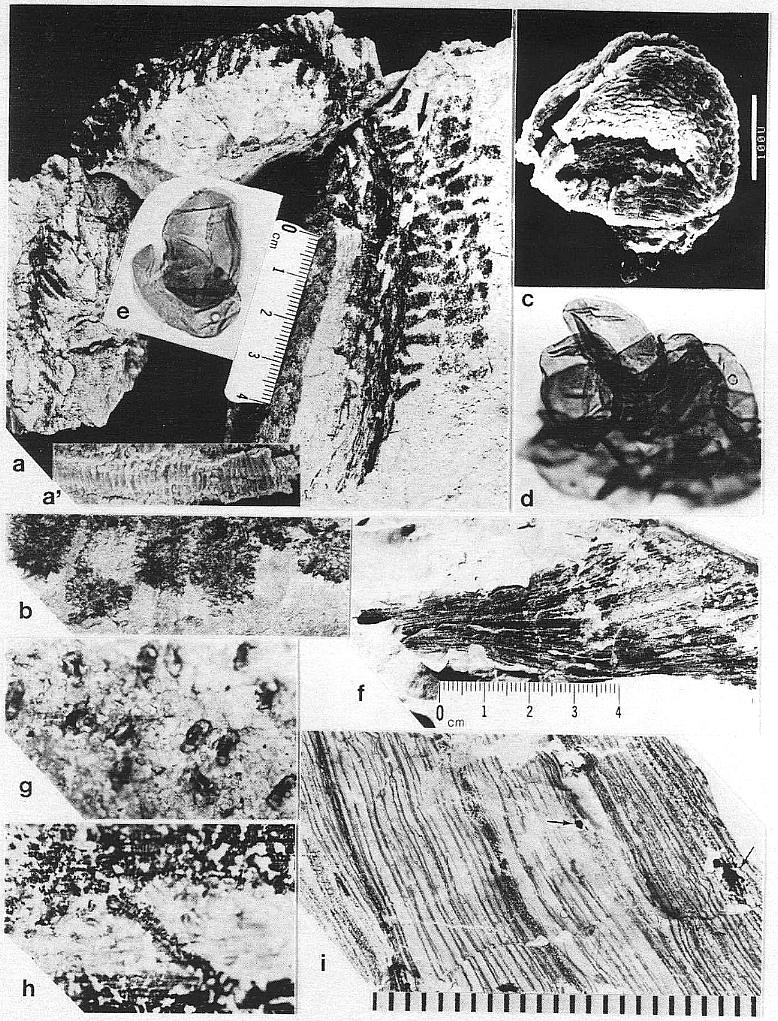
DESCRIPTION: One nearly complete reproductive axis and two isolated portions bearing reproductive organs were found in close proximity to one another. The most complete axis is 48.5 cm long, and was recovered in three large pieces, with a smaller piece of the main axis from the break about midway along the specimen (Pl. 5, fig. f; Pl. 6, figs. a-c). A small piece (est. 4 cm) of main axis from the middle of the specimen was lost during recovery, and the tip of the main axis is missing (Pl. 6, fig. a). Since its base above the attachment of the last spathe-like vegetative leaf (Pl. 5, fig. f) was not recovered due to excessive overburden, the main axis probably exceeded 53 cm in length - the block with the leaf (PP34319) was the last part of the inflorescence removed from the outcrop, while the block with the reproductive organs (PP34316) was the first part discovered.
A large plicate Sanmiguelia-type leaf obscures the basal part of the main reproductive axis and any bracts it may have borne (Pl. 6, fig. f). The distal part of the leaf is 16 cm long with a maximum folded width of 5.5 cm. The leaf is folded in half, with its adaxial side facing the reproductive axis as though the leaf joined the axis below the base of the specimen. The leaf apex is incomplete and slightly frayed as is typical of Sanmiguelia lewisii (Tidwell et al., 1977). Parallel veins are present, but they are not as well preserved as in other leaves from the same outcrop.
The lower half of the main axis above the leaf is nearly naked, bearing only four small bracts of increasing size distally. These bracts are spirally borne, parallel-veined, distally acuminate, and the distance between them decreases distally: 5 cm, 3 cm, and 2.8 cm, respectively. The next bracts in sequence have progressively longer sheathing leaf bases and longer more tapered leaf blades (Pl. 6, figs. b and a). Six bracts or cataphylls occur along the lower part of the upper half of the main axis before reproductive organs are found clustered about the axis. They have parallel veins that converge and fuse apically. The sheathing leaf base of the last of the six cataphylls increases in breadth, broadening upwards as a secondary branch diverges from the main axis (Pl. 6, fig. a, ax2). Sheathing leaf bases of subsequent cataphylls could be recognized along the main axis, because sediment accumulated between them. Through degaging, at least two leaf bases were found to overlap before the carbonaceous residue around a sediment-filled pith cast was encountered.
Four cataphylls of decreasing size occur along the main axis above the level where reproductive organs first occur. These cataphylls, along with the largest one below them, have portions of secondary axes lying next to them and diverging from the main axis (Pl. 6, fig. a, ax2). The secondary axes appear to have several parallel folds or grooves running along them (Pl. 6, fig. g), and in cross section appear to have been five or six sided. The free end of a small scale bract was observed attached to one of them; scale bracts may characterize the secondary branches, but they were probably widely spaced (see figure 7c for an interpretation), since the visible portions of secondary branches rarely possess them. The occurrence of mainly short segments of secondary branches suggests that they were relatively rigid in life, and were broken and fragmented during burial or compaction of the sediments. The secondary axes appear to have emerged from between the sheathing bases of successive cataphylls, and their spacing in the sediment around the main axis suggests one branch per cataphyll. These axes can be distinguished from associated fern rachises by their parallel folds or grooves, and by the absence of attached fern pinnules.
The main axis of Axelrodia burgeri sp. nov. ends with a relatlvely short segment of axis (at least 3 cm in length, but incomplete in Pl. 6, fig. a) bearing small spirally arranged scale bracts. This portion of the main axis is slightly wider than the secondary axes, there are no visible grooves, and the scale bracts are more closely spaced than on secondary branches. The reproductive axis appears to have terminated through unequal vascular division, decreasing in width with each emerging secondary branch, but retaining the potential for further growth (i.e. indeterminate growth).
A small but presumably variable number of tertiary branches arise from secondary branches; because of the fragmentary preservation of the secondaries, only proximally or distally-borne tertiary branches can be documented (Pl. 6, fig. k, ax3 adjacent to ax2; see also figure 7c for an interpretation). Terminal reproductive organs typically occur within 5-8 mm of the secondary branches (Pl. 6, fig. a), indicating that tertiary branches are short, and explaining why they are difficult to recognize. Two to three megasporophylls and associated bracts are usually found together in a cluster, suggesting that the tertiary branches divide ultimately almost immediately after diverging from the secondary branches (cf. Pl. 6, figs. g and k). Each megasporophyll-bract unit is subtended by a long (21-24 mm minimum length), abaxially-curved conduplicate bract (Pl. 6, figs. g-h, b2).
Megasporophylls and Associated Structures on Tertiary Branches
The reproductive organs borne on tertiary branches are solitary carpel-like megasporophylls, each of which has its own complement of bracts. Because all the megasporophylls are alike, whether they are borne independently on tertiary branches or in clusters at the ends of secondary branches, they will be described separately below. The enclosing bracts are also similar, but are borne differently for solitary and aggregate megasporophylls. Each solitary megasporophyll is enclosed by at least two tepal-like bracts, which join and fuse with the base of the megasporophyll. Preserved stages of their development indicate that they did not enlarge as rapidly as the maturing megasporophyll (compare figures Sa and 5e), reaching a maximum length of 12-15 mm. These bracts may also have been basally joined or connate, as is hinted at in the only example where two inner bracts (p1) are clearly shown (Pl. 6, fig. h; figure 5e) after the removal of the lower half of the megasporophyll (ms). In most cases, the solitary megasporophyll covers or hides one of these bracts.
The inner bracts are covered with long multicellular(?) hairs, similar to those that cover the megasporophylls (Pl. 7, fig. c). The hairs are easily visible at a magnification of 4X, but cellular details are unclear. The hairs tend to be oriented in the direction of the bract (figure 5), and frequently diverge outward into the enclosing sediment. The cuticles of the inner bracts, like those of the megasporophylls, are thin and poorly preserved, leaving the thick-walled hairs attached to an organic film that is frequen~ly nQn-recoverable and destroyed upon acid maceration. There is no evidence of fibers preserved beneath the cuticles.
The inner bracts are partially covered or overlapped by a pair of outer bracts, which appear to join the base of a solitary megasporo,phyll on opposite sides (Pl. 6, fig. h; figure 5e). The inner and outer bracts form whorls, and it appears that the outer bracts alternate with the inner ones, although this is not particularly clear in the specimens. The outer bracts are much larger t,h.n the inner bracts, and divide upwards near their base into five or six long, 1.5-2.0 mm-wide strap-shaped bands, each of which possesses a stiff central vein (Pl. 6, fig. h). In most cases, tbe outer bracts (p2) can be seen as subparallel to curved bands radiating away from megasporophyll subunits to one side of the inflorescence axis (Pl. 6, fig. a; Pl. 8, figs. b-c); the digitate morphology and disposition of the outer bract8 is preserved around an immature or aborted megasporophyll in that specimen (ms* in Pl. 6, fig. a; Pl. 8, fig. c). The free distal bands may reach a length of 20 mm or more, and appear to arise about 4-5 mm above the united base of the bract (these dimensions relate only to mature or nearly mature bracts; figures 5a, 5e, and 5g). During the development of the megasporophyll, the outer bracts may have wilted and fallen off, since they seem to be absent around nearly mature solitary megasporophylls, and sometimes can be found bent downwards away from those megasporophylls (e.g. figures Sa and also 7c for a reconstruction).
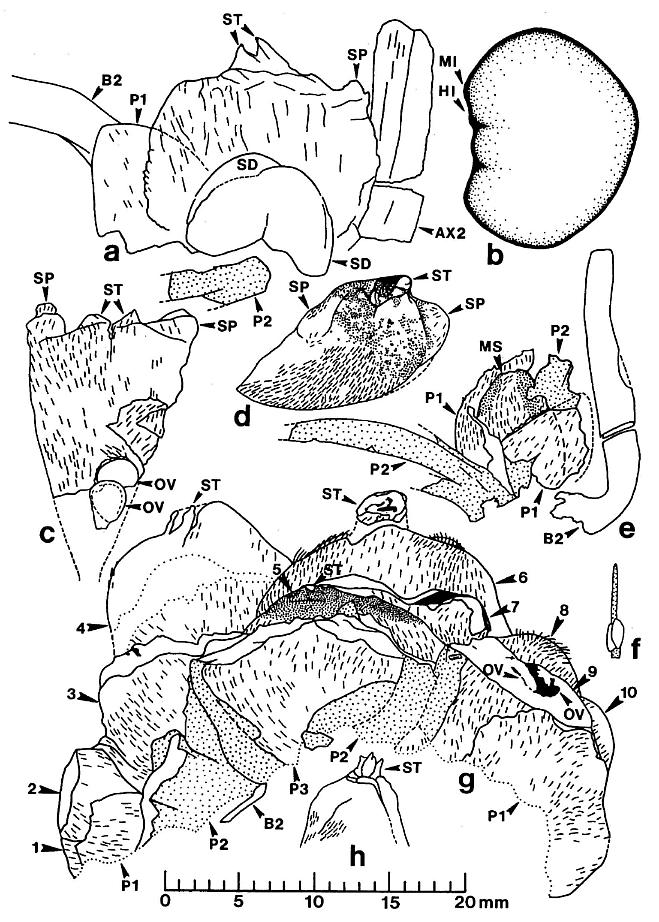
Figure 5. a-h. Camera lucida line drawings of Sanmiguelia lewisii megasporophylls and restoration of dispersed seed; all drawings to same scale. -a. Axelrodia burgeri, nearly mature, solitary megasporophyll unit containing two developing seeds (Pl. 6, fig. g). -b. Nemececkigone fabaforma, dispersed seed (Pl. 6, figs. e-f). -c. A. burgeri, immature isolated megasporophyll containing two ovules (Pl. 6, fig. c). -d. A. burgeri, megasporophyll from a cluster terminating secondary branch of inflorescence (Pl. 6, fig. i). -e. A. burgeri, solitary megasporophyll borne on tertiary branch, showing three types of associated bracts (Pl. 6, fig. h). -f. A. burgeri, stamen-like organ found adjacent to specimen in figure 5e (Pl. 6, fig. h, an). -g. A. burgeri, flower-like reproductive unit terminating secondary branch, showing ten megasporophylls, mostly sediment-filled, some with visible ovules or developing seeds inside (Pl. 6, fig. k; also Pl. 7, figs. d-e). -h. A. burgeri, apex to one of the megasporophyll in a secondary branch cluster (Pl. 7, figs. e and j), showing possible veins in stigma-like apex and internal impression of suture along one side. Orientation and preserved density of hairs given on all specimens. Outer digitate tepals given dot pattern. Carbonaceous inclusions or structures shown in black. AX2, secondary axis; B2, subtending conduplicate bract; HI, hilum; MI, micropylar pit; MS, immature megasporophyll; OV, ovule or developing seed; PI, inner hairy bract of perianth; P2, outer glabrous bract of perianth; SD, immature seed; SP, shoulder pocket on lateral side of megasporophyll; ST, bilobed stigma-like apex to megasporophyll.
The outer bracts can be distinguished from the inner ones even as incomplete segments because of their glabrous cuticle, which shows no signs of epidermal hairs. Even though the cuticle is thicker, it does not preserve much better than that of the inner bracts, and it was evidently only thinly cutinized. Consequently, acid maceration produces very few identifiable fragments of cuticle from these bracts. The contrasting difference between inner and outer bract cuticles made it possible to trace or follow these structures under magnification, even where they overlapped (cf. figure 5).
In addition to the perianth, one isolated stamen-like organ with a laminar base and long filamentous apex was found intimately associated with portions of two megasporophylls and their bracts (Pl. 6, fig. h, an; figure Sf). Dissection of the two elliptical bodies joined to the filament produced only ovoid cells similar in size to the pollen of Synangispadixis tidwellii sp. nov. The distal filament to the stamen-like organ (not visible in the photograph) is oriented subparallel to the digitate bands of the outer bracts, and is directed away from the basal portion to a second megasporophyll (Pl. 6, fig. h); this association-orientation raises the possibility, at least, that the megasporophylls and their associated bracts were either bisexual, or possessed staminodia - an indication of an ancestral bisexual condition. Since no other stamen-like organs were found, the identity and attachment of this organ to the outer bracts must remain conjectural at this time. More information concerning the possible manner of attachment of such an organ will be addressed in the description below of clusters of megasporophylls and their perianth-like parts terminating secondary branches.
Clusters of Megasporophylls on Secondary Branches
The flower-like reproductive units borne terminally on secondary branches of the inflorescence consist of clusters of carpel-like megasporophylls surrounded by a perianth-like structure composed of bracts (Pl. 6, figs. k-l; Pl. 7, figs. d-e; figure 5g and also figure 7c for a reconstruction). The number of megasporophylls, and consequently the size of the clusters, may vary; along the distal portion of the inflorescence axis (Pl. 6, fig. a) these units appear as compressed and distorted masses of overlapping megasporophylls and bracts. Some of the flower-like units appear to have no more than ten megasporophylls; however, it is difficult to impossible to distinguish the outlines and identities of all parts, since most of the megasporophylls collapsed either because they were damaged or before they could be filled with sediment. Some of them broke apart during burial and compaction, yielding isolated megasporophylls (Pl. 6, fig. c; Pl. 8, fig. b, large arrows). An informative specimen of a flower-like unit, however, was fossilized after the megasporophylls became filled with clay and silt, which preserved the integrity and shape of some carpel-like parts.
The apical flower-like unit had from ten to eighteen carpel-like megasporophylls at its center, but may have had more. An isolated specimen preserved in three dimensions (Pl. 6, figs. k-l) will serve as the example: Unlike the solitary megasporophylls borne on tertiary branches, the megasporophylls of the flower-like unit appear to lack individual bracts. Instead, they are collectively surrounded by an estimated eight or nine inner bracts, some of which are hairy and others of which are glabrous. There is also the possibility that some of the outermost megasporophylls alternated with the bracts, since an ordered phyllotaxy of individual whorls is not evident. The inner series of bracts in turn is surrounded by three or four conduplicate bracts (see figure 7c for an interpretation). Sediment fills the gaps between the perianth-like parts, and allows them to be individually followed (Pl. 7, fig. d). Most of the hollow megasporo- phylls were filled with sediment, and some were transversely broken. Cross sections of megasporophylls can be distinguished from the hairy bracts by the manner in which their walls completely surround a central chamber, and by the remains of ovules or seeds preserved within those chambers (Pl. 6, fig. k; Pl. 7, fig. e; figure 5g).
All of the floral parts appear to be attached to a receptacle, or swollen secondary branch apex (Pl. 6, fig. 1). The hairy simple bracts and blabrous digitate bracts apparently alternated with one another, forming a close spiral resembling a perianth around a cluster of megasporophylls (figure 5g). This change in arrangement bring~ some of the digitate bracts into contact with the megasporophylls, a condition not observed for megasporophylls on tertiary branches. In addition, some hairy simple bracts overlap and lie outside the glabrous digitate bracts. The exact number of bracts cannot be determined from the available specimens, but three hairy bracts and two glabrous bracts can be clearly distinguished on one side of the flower-like unit (figure 5g and also figure 7c for a reconstruction).
The digitate part or strap-like bands of the glabrous bracts are not preserved in the portion of the specimen recovered (Pl. 6, fig. k; Pl. 7, figs. d-e). The bases of those bands, however, are preserved and appear as vertical folds and overlapping flaps of cuticle (figure 5g). The glabrous bracts possess an addition- al flap of tissue on their basal adaxial side, which is not identifiable as one of the strap-like bands (figure 5g, p2). This flap was not identified on the bracts associated with solitary megasporophylls, probably because of insufficient material. The isolated stamen-like organ (staminodium?) found associated with a pair of solitary megasporophylls (Pl. 6, fig. h; figures 5e-5f) may have been attached to such a flap. Until more specimens are found and the identity of this flap is established, its function and significance remain obscure. Therefore, the reconstruction in figure 6c shows the flap only as it is preserved in the specimen.
The Megasporophyll
The megasporophylls have a narrow base that expands upwards to a broad rounded apex possessing a terminal stigma-like process (Pl. 6, figs. a and i; figures 5c and 5d). The megasporophylls are covered with hairs 400-450 um long, and show numerous small to large reddish-orange to black resin bodies scattered across the apical surface where the hairs have been removed (Pl. 6, fig. i; figure 5d). The hairs are usually well preserved, but the underlying cuticle is thin. There is no apparent suture or opening along the sides of the megasporophyll, but the apical stigma-like process contains a central canal or opening flanked by a u-shaped collar (Pl. 6, fig. j) that projects slightly above the top of the megasporophyll body. On top of this collar are two conical flaps of tissue (Pl. 6, figs. c and i; Pl. 7, figs. k and j; figures 5a, 5c, 5d, 5g, and 5h, st). On the inside of the stylar-like canal are carbonaceous folds or strands that radiate upwards and outwards (figures 5d and 5h); these strands may represent the remnants of vascular traces. The outer hairy cuticle appears to continue onto the stigma-like process, where it joins a non-hairy cuticle surrounding the inside of the apical canal (Pl. 6, fig. j).
The canal is tear-drop shaped with the pointed end forming a short suture on one side; a fold or groove representing the con- tinuation of the suture is visible along one side of an internal cast of the megasporophyll locule (Pl. 7, fig. j, arrows; figure 5h). Whether the suture is oriented abaxially or adaxially is uncertain, but it is not lateral, because of the presence of shoulder pockets or projections (sp) on either side of the stigma-like process (Pl. 6, fig. c; figure 5c).
The ovules or seeds in many megasporophylls are obscured by overlapping bracts or other megasporophylls. Where visible, however, a pair of ovules or seeds is attached to one wall of the ovary (Pl. 6, fig. k; Pl. 7, fig. d, ov; figure 5g, ms #9). The smallest megasporophyll observed is about 7 mm long (Pl. 6, fig. a, ms*), while the largest are about 17 mm long (Pl. 6, fig. g; Pl. 7, fig. e; figure 5g). The height of the megasporophyll increased rapidly at first, followed by a progressive increase in breadth, as a graph of height versus two-dimensional size demonstrates (figure 6). Ovules are difficult to recognize in megasporophylls 14 mm or shorter. The smallest megasporophyll with recognizable ovules is about 15 mm long, and possesses a pair of anatropous ovules near its base (Pl. 6, fig. c; figure 5c, ov). At this stage of development the ovules are only 2 mm long, and the megasporophyll has developed sizeable shoulder pockets (sp) of expanded wall tissue, indicating intercalary growth comparable to that of Sanmiguelia leaves (figures 5c and 5d). The ovarian chamber at this stage is estimated to have been 100 times the volume of both ovules.
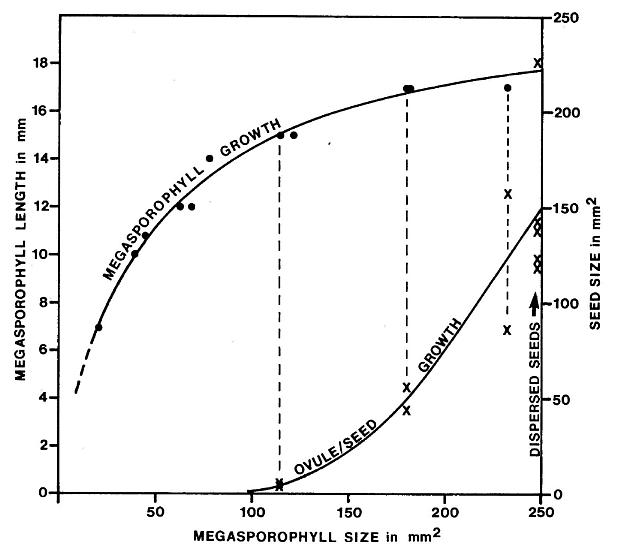
Figure 6. Growth relationships between carpel-like megasporophylls and enclosed ovules or seeds of Axelrodia burgeri, with dispersed seeds (Nemececkigone fabaforma) added for comparison. Measured specimens include holotype material (Pl. 6, figs. a-b, e-f). Two dimensional area measured by placing a grid over outlines of specimens drawn from photographs; some reconstruction or estimate of size was involved for either incomplete specimens or partially hidden specimens. Not all larger megasporophylls had enclosed seeds which could be measured.
A nearly-mature 17 mm-long megasporophyll contains the compressions of two overlapping ovoid ovules or seeds measuring about 7-8 mm in diameter (Pl. 6, fig. g; figure Sa). These ovules/seeds are positioned near the base of the ovary. The ovarian chamber is about 90% larger than the previous example (figure 5c), while the ovules or seeds are about 350% larger. Another megasporophyll (PL. 7, fig. e; figure Sa, ms #4) contains two nearly mature, bean-shaped seeds of unequal size (identical to Nemececkigone fabaforma sp. nov.; see below), measuring 11 mm by 14 mm and 8 mm by 9 mm. The ovarian chamber is only about 3% larger, while the seeds are about 140% larger than the previous example, and completely fill the ovary. In addition, a smooth seed coat is indicated like that for the dispersed seeds, which was resistant to compaction during burial. Although the number of examples showing ovule/seed development is small, the implications are not (figure 6); see below:
DISCUSSION: For reasons presented later, the closed suture along one side of the megasporophyll is considered to be the union of opposite sides of a sheathing leaf base that encircled an ovule-bearing axis, and would therefore be located in a ventral position relative to the floral axis. According to this interpretation, the apical stigma-like process would be either a much reduced leaf blade or a pair of stipules flanking an aborted leaf blade apex (see figure 10 for a comparison of possible homologous organs and their parts).
The hollow nature of the megasporophyll is demonstrated by specimens in which the locule is filled with sediment (Pl. 6, fig. k; Pl. 7, figs. d-e). A number of compressed megasporophylls also contain some sediment - usually in the form of seeds, which resisted compaction long enough during decay to be either partly or completely replaced by clay casts (e.g. Pl. 7, figs. e and j, sd). Sediment-filled megasporophylls contain a finer more clayey matrix than that which surrounds them, suggesting a post-depositional migration of fines. The sediment-filled ovary indicates not only that there was an open stylar-like apical canal, but that the walls of the megasporophyll were originally rigid or thick enough to resist collapse before the sediment entered. Those megasporophylls that lack internal casts show signs of being damaged or torn, but even as compressions there is no thick carbonaceous residue present. The loss of cell turgor (due to injury) shortly after burial may explain why some mega- sporophylls collapsed and others did not. The ovary appears to have contained only ovules or seeds, because any other type of tissue would have either blocked sediment entry or left a trace of its former existence. Furthermore, the manner in which the ovules and seeds developed precludes the existence of any type of growth-restraining tissue.
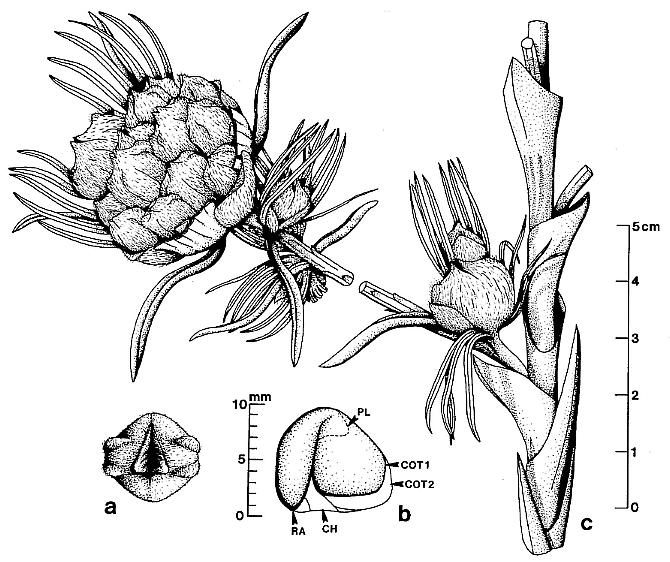
Figure 7. Reconstructions of Axelrodia burgeri sp. nov. and an embryo found inside Nemececkigone fabaforma sp. nov., the probable dispersed seed of Axelrodia. -a. A. burgeri, distal view of a carpel-like megasporophyll, showing apical bilobed stigma-like process with its central stylar-like canal and expanded shoulder pockets representing active intercalary meristems "anticipating" the development of enclosed seeds. -b. N. fabaforma, morphology of an enclosed embryo, showing two prominent cotyledons, one of which is smaller than the other, and a radicle, hypocotyl, and plumule, all of which have recognizable features in the embryo cast (Pl. 7, fig. 1). -c. A. burgeri, distal portion of inflorescence, showing primary branch with its long sheathing cataphylls, and one secondary branch with its terminal flower-like reproductive unit and solitary megasporophylls borne in groups on tertiary branches; those organs or characteristics which are imperfectly known are either omitted (e.g. stamen-like organs) or not shown clearly (e.g. base of receptacle; length of secondary branch). Smaller scale for a. and b.; larger scale for c. CR, chalaza; COT1, smaller cotyledon on flatter side of seed; COT2, larger cotyledon filling convex side of seed; PL, plumule.
The megasporophyll apparently developed much like that of an angiosperm carpel, enlarging to form an ovary many times the size of the ovules; the ovules/seeds did not begin to develop until the megasporophyll had reached about 40% of its mature size. Once the ovules began to enlarge, they increased exponentially in size to fill the ovary. The reason for the delay in ovule development is conjectural, but fertilization may have been required before development took place as in angiosperms. The morphology of dispersed seeds, including an embryo cast, indicates that there was no functional micropyle at maturity, the micropylar pit lay adjacent to the hilum, seeds developed in symmetrical pairs with left and right-handed symmetry, and the embryo had two cotyledons, one of which was significantly smaller than the other.
The hairy simple bracts surrounding solitary megasporophylls may have provided a protective function during early development, delaying the time of anthesis until they opened. The identical cuticular morphology of the megasporophyll and inner bract indicates that both may have developed phylogenetically from leaf-like organs. The glabrous digitate bracts of the outer perianth may have functioned more as wind baffles than for protection or insect attraction. Their stiff flat lobes projecting upwards and above the megasporophyll could be compared to the pre-integumentary lobes of some Paleozoic cupules, which Niklas (1981) has demonstrated functioned to control air flow over the ovule. Changes in airflow pattern caused by the lobes increased the incidence of pseudopollen landing near or on the micropylar opening of his models. If the reconstructions in figures 7 and 8 are accurate and the above interpretation is correct, the morphology of the digitate bracts surrounding solitary and aggregate megasporophylls correlates with the anemophilous morphology of associated pollen-bearing axes (i.e. Synangispadixis tidwellii sp. nov.). Whether or not the resin bodies and glandular hairs observed on the megasporophylls indicate a food source (e.g. nectar drops) for insects is conjectural, but a plant that relied on more than one agent for pollination (ambophily) would have had an increased chance of survival over anemophilous competitors in the Late Triassic.
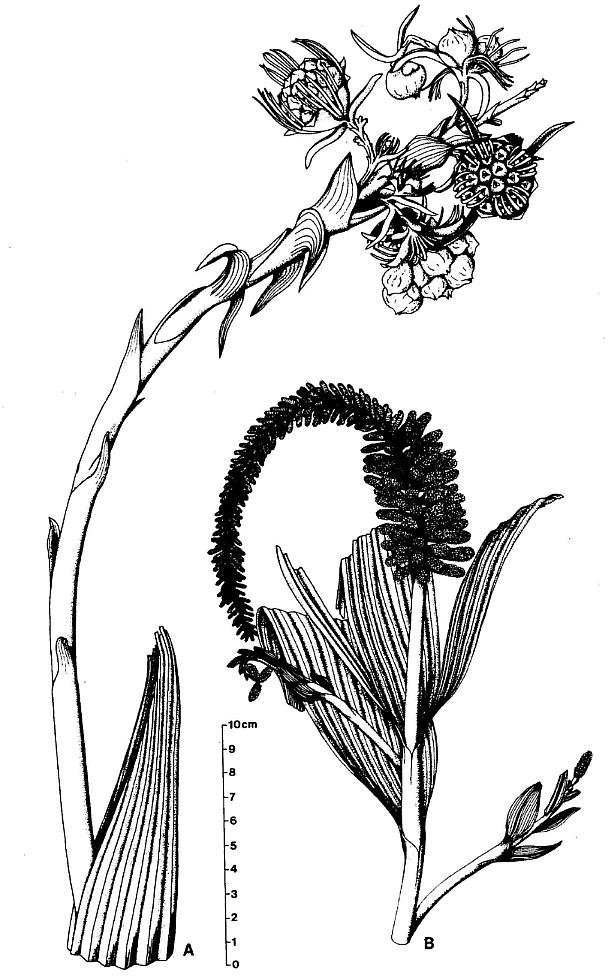
Figure 8. Reconstructions of Axelrodia burgeri sp. nov. and Synangispadixis tidwellii sp. nov. attached to stems bearing Sanmiguelia lewisii leaves. -a. A. burgeri, based on holotype (Pl. 5, fig. f; Pl. 6, figs. a-b), showing inflorescence axis emerging from between the folds of a large spathe-like leaf. -b. S. tidwellii, based on holotype (Pl. 3, fig. a), hypotype (Pl. 4, fig. a) and related specimens (Pl. 5, figs. a-c, d), showing presumed relationships between spadix-like inflorescence and a terminal spathe-like leaf, subtended on the stem by secondary branches in the axils of leaves, which terminate in one or more synangia-like organs that are probably homologs of secondary branches on the main inflorescence.
THE PROBABLE DISPERSED SEEDS OF SANMIGUELIA
Nemececkigone Cornet gen. nov.
TYPE SPECIES: Nemececkigone fabaforma Cornet sp. nov.
DIAGNOSIS: Large to very large, elliptical to bean-shaped seed typically 14 mm long-II mm tall-8 mm wide with a smooth outer surface, a prominent proximal (chalazal) scar, and a micropylar pit at one end adjacent to hilum. One side of seed convex, rounded, the other significantly less convex or flat, with a median crest or ridge separating both halves. The median ridge is developed to varying degrees around the perimeter of the seed, forming a suture proximally, which intersects hilum on side of chalaza lacking the micropylar pit. Seed coat thin, with faint striate pattern on inner surface, hard, resistant to compression, splitting open along median ridge or suture into two unequal halves, which are occasionally found separately in sediment. Seeds possess either right or left-handed symmetry, with flatter side reversing position. Embryo cast indicating root cap, hypocotyl, plumule, and two fleshy cotyledons, one of which is significantly larger than the other, the larger cotyledon on the more convex side of seed.
DERIVATION: Nemececki - after Anthony Nemececk (deceased, 1980), an old friend and horticulturist who gave the author his first botany lesson as a child, and whose love of flowers and seeds influenced the author towards a professional career in botany; gone - Greek, meaning seed.
DISCUSSION: This name is intended for dispersed seeds fitting the above diagnosis found associated with the remains of Sanmiguelia spp. or found in rocks of the same general age as Sanmiguelia. Even though similar seeds occur in megasporophylls of Axelrodia, the origin of dispersed seeds remains speculative. Therefore, a separate name is required.
Nemececkigone fabaforma Cornet sp. nov.
HOLOTYPE:
PP34329.
HYPOTYPE: PP34330-PP34332; PP34335.
DIAGNOSIS: As for the genus.
REFERENCES: 1972 Globose to orbicular seed-like objects and possible fruits associated with the remains of Sanmiguelia lewisii, Becker, Palaeontogr. V. 138B, pp. 181-185, Pl. 37, figs. 1, 2.
OTHER MATERIAL: PP34333-PP34334.
RELATED MATERIAL: PP34321.
NUMBER OF SPECIMENS EXAMINED: 8 dispersed; 2 in PP34321.
ILLUSTRATIONS: Pl. 6, figs. d, e, f; Pl. 7, figs. e, f, g, h, i, j, 1; figures 5b, 7b.
DERIVATION: From faba - Latin, meaning bean; forma - Latin, meaning form; fabaforma - meaning bean-like, bean-form.
DESCRIPTION: The seeds are found mostly as sediment-filled casts with an organic-stained or thin carbonaceous outer wall (Pl. 6, figs. d-f; Pl. 7, figs. f-h). They have also been found as isolated seed coats representing one of the halves of the seed (Pl. 7, fig. i). One side of the seed is invariably flatter than the other, giving the seed a clam-shell shape. Viewed from the convex side, the seed looks like a large lima bean. Viewed from the chalazal side, the seed is wider at one end, tapering towards the narrow end. There is a prominent round scar or hilum posi- tioned eccentrically towards the narrow end of the chalaza (Pl. 6, fig. d; Pl. 7, fig. f). Adjacent to this scar at the narrow end of the seed is a small pit representing the micropyle; the pit could be clearly seen on only two specimens (Pl. 6, fig. d; Pl. 7, fig. f, arrow), and was obscured or destroyed on the others. There was no structure at this pit which would indicate that it was open or that a micropylar tube extended from it. The suture between the two halves was apparently weaker proximally adjacent to the hilum, because compression of the seed during burial sometimes resulted in the two halves of the seed coat separating and curling inward adjacent to the hilum (Pl. 7, fig. f).
The dispersed seeds range in size from 10 mm long-10 mm tall-8 mm wide to 18 mm 1ong-15 mm tall-9 mm wide. The majority are longer than tall with the more common size being 14 mm long-11 mm tall-8 mm wide. The seeds seem to fall into two size classes: Medium to small seeds 15 mm or less in length, and large seeds 16 mm or longer (figure 6). A pair of seeds identical to Nemececkigone fabaforma sp. nov. and possessing their characteristic median ridge or keel (Pl. 7, figs. e and j, kl) was found inside a carpel-like megasporophyll of Axelrodia burgeri sp. nov. These seeds are of two distinctly different sizes (figure 6; Pl. 7, fig. e, sd), indicating that extreme differences in size are either developmental or adaptive, but not specific.
The seed-like bodies described by Becker (1972) from the type locality for Sanmiguelia are similar to those of Nemececkigone fabaforma sp. nov., and are of variable size: 7-12 mm in length by 5-10 mm in width. These seeds fall into the smaller size class for the Sunday Canyon material. They also show indications of wall striations, as well as a smooth seed coat.
In addition to seed casts and isolated seed coats, two embryo casts were found (Pl. 7, fig. 1; figure 7b). One of the halves of the seed coat apparently came off during burial, al- lowing sediment to quickly surround the delicate tissues of the embryo. In one specimen the clay cast three-dimensionally separate and distinguish the collapsed carbonaceous remains of two cotyledon-like organs, a plumule or epicotyl between the bases of the cotyledons, an elongate hypocotyl, and a radicle or root cap next to the hilum where the micropyle would have been (figure 7b). The cotyledons are of distinctly different sizes, with the larger one filling the convex side of the seed, and the smaller one tucked in the curl of the larger cotyledon. The large amount of carbonaceous residue and the size of the casts suggest that the cotyledons were fleshy.
DISCUSSION: The presence of similar seeds at both the type locality in Colorado and at Sunday Canyon in Texas supports the interpretation that the fossils described in this paper belong to one plant, Sanmiguelia lewisii Brown. Only with additional data will it be known whether these seeds are peculiar only to Axelrodia and Sanmiguelia. A bean-shaped seed with one side flatter than the other, and a micropylar pit adjacent to the hilum might suggest the presence of a u-shaped dicotyledonous embryo with one cotyledon smaller than the other. The embryo cast demonstrates that the superficial characteristics of the seed reflect the general morphology of the embryo.
THE HABITAT AND HISTORY OF ONE SANMIGUELIA COLONY
The sedimentary sequence in which Sanmiguelia is preserved indicates changing habitat conditions. The sequence begins with a paleosol, in which the vertical stems are firmly anchored by roots; primary and secondary roots can be traced downwards at least 8 cm (figure 2). Three clusters of two to four vertical axes were uncovered during excavation. They were spaced 175 cm and 85 cm apart. Two clusters are illustrated in figures 2a and 2b. The paleosol gradually rises in elevation to the west, where it is truncated by a channel fill sequence (figure 1). Several ferns were preserved adjacent to the westernmost cluster of axes. Their fronds were found on top of the paleosol, and fern rhizomes and roots could be followed in the paleosol. Only one type of fern leaf was found, which is assignable to Cladophlebis cf. C. macrophylla Fontaine. Associated with the sterile fronds were fertile spikes containing thousands of spores assignable to Cyclotriletes margaritatus Mädler (1964).
Above the paleosol a 1-5 cm thick layer of dark gray shale could be followed for a short distance down slope, where the shale is replaced by mudstone. C. margaritatus is the most common dispersed spore in the shale, even though a diversity of other spore types is also present. The shale thickens and thins as the overlying layer of pinkish-buff siltstone (layer Sl in figure 2) intrudes down into it. In some places siltstone loadcasts distort and deform the underlying shale layer, and also the fossil plants the shale contains. Within the shale well-preserved plicate leaves assignable to Sanmiguelia were found. Many of these leaves were large, although they were frequently folded and ripped either during burial or during rapid loading of the soft mud matrix by the overlying siltstone, resulting in uneven compaction and diaperic movement (Pl. 1, fig. c). Post-depositional movement is evidenced by very small slip surfaces or faults displacing portions of the same leaf.
The pinkish-buff siltstone layer (S1 in figure 2) above the shale varies from 3 cm to 5 cm in thickness. Some of the verti- cal axes ended abruptly at the top of layer S1, while others continued through it. Layer S1 is overlain by a 14-18 cm thick gray sha1ey siltstone layer (S2), which becomes a dark gray shale to the west (up depositional dip), and a gray mudstone to the east. Three vertical stems ended abruptly at the top of layer S2, while at least two stems were followed upwards to the overlying conglomeritic sandstone. Two pinkish-buff to grayish-buff sandy siltstone layers (S3 and S4) overlie layer S2. A depositional surface separating these two layers was recognized by bends in the vertical axes that pass through them. One of the axes appears to have terminated at this surface, with a side branch continuing the upwards growth of the plant (Pl. 2, fig. b).
A cluster of Sanmiguelia leaves was found terminating a vertical axis at the top of the siltstone sequence by Paul E. Olsen (1981, personal communication), and deposited in the Yale Peabody Museum collection. The apical leaves were reported by Olsen to be smaller than the leaves lower on that axis, as Tidwell et a1. (1977) noted for their Colorado specimens. From about the same stratigraphic position another leaf was found overlapping a specimen of Synangispadixis tidweii sp. nov. (Pl. 4, fig. a). Both of these specimens suggest that the stems did not extend much higher. Tidwell et al. (1977) report axes measuring at least 61 cm in height. The maximum length of stems preserved at the Sunday Canyon locality is 65 cm.
The environment in which Sanmiguelia grew appears to have bordered a meandering river channel (Olsen, 1985). Initially, a colony of Sanmiguelia grew side by side with ferns in soft water- soaked soil at the edge of an interdistributary lake or pond. Soon after the colony established itself through an extensive rhizome system, the margin of the lake began to encroach upon the colony, and an organic-rich mud layer was deposited on top of the paleosol. The ferns were apparently killed off by the rising water, but Sanmiguelia continued to grow. Some wind(?) torn leaves fell off the stems into the mud, where they were preserved along with the ferns.
The first pinkish-buff siltstone layer (S1) was probably deposited by a turbidity current distal to a crevasse splay during a flood (Olsen, 1985). Some large stems bearing an Axelrodia burgeri sp. nov. inflorescence were knocked down by this high energy event, and were found sandwiched between the siltstone and the underlying dark gray shale. Either rising water or flood damage ended the growth of some of the vertical axes, while others continued to grow and repair themselves. Those axes that died were broken off before or during the next flood, while new shoots propagated upwards from the bases of damaged stems or from rhizomes. Each successive flood added a new layer of sediment (S1-S4, figure 2), and with each deposit some stems were irreparably damaged. Many of Sanmiguelia's leaves were ripped and torn off during the floods, and their fragments can be found randomly distributed throughout the siltstone layers. With the loss of leaves, continued growth and survival depended on the preservation of apical and lateral shoot meristems, which were probably protected by sheathing leaf bases. Its sturdy stems, regenerative capacity, and growth along the margins of a lake indicate that Sanmiguelia was probably well adapted for climatic extremes in a fluvial red bed environment frequented by floods.
COMPARISONS WITH FOSSIL PLANTS
There are several fossil plants described in the literature that are similar to Sanmiguelia and its probable reproductive structures: The secondary axes of Synangispadixis tidwellii sp. nov. with their aggregation of sporangia (microsporophylls) compare with the microsporangiophores of the latest Triassic Irania hermaphroditica (Schweitzer, 1977), which are borne below megasporophylls on the same axis. Unlike Synangispadixis, however, the microsporangia are stalked and the stalks terminate with two suspended ellipsoidal pollen sacs. The megasporophylls are spirally arranged and consist of a pair of capsules at the ends of a dichotomized branch. Each capsule consists of a pair of basally joined megasporophylls bearing about six ovules each on their adaxial or facing sides. The morphology of the megasporangiophores differs considerably from the megasporophylls and associated structures of Axelrodia burgeri sp. nov. The synangia-like morphology of the microsporangiophores and the paired decussate morphology of the megasporangiophores in Irania compare more with similar structures in the Gnetales (e.g. Ephedra) than with Synangispadixis, Axelrodia, or the angiosperms. In addition, Desmiophyllum armanii, the leaves associated with Irania, have parallel veins that end blindly at the distal margin, and show no tendency toward apical vein fusion as in Sanmiguelia.
Terminal seed-bearing axes that superficially resemble large gymnospermous cones have been described from the Richmond Basin coal measures of Virginia. The Richmond Basin sequence has been more recently dated as early to middle Carnian in age (Cornet and Olsen, 1985). Fontaine (1883) originally described these axes under the name, Zamiostrobus virginiensis, and considered them to be cycad cones (Fontaine, 1883, p. 85, Pl. XLVII, figs. 4, 5). Bock (1954) renamed them, Primaraucaria wielandii, and compared them to the cones of araucarian conifers. Later, Bock (1969) reported finding about 200 specimens, and indicated that they were a common element in the Winter pock coal flora, where they were associated with Podozamites tenuistriatus Fontaine, large Macrotaeniopteris and Eoginkgoites leaves, large fern fronds, and giant Equisetites stems.
Four specimens of these fruits were obtained from the Philadelphia Academy of Natural Sciences, where Bock had deposited them. The most striking evidence against a coniferalean affinity is their non-woody stems and reproductive axes that lack a carbonaceous core (Pl. 9, figs. b, d, and h). Large ovoid compound fruits are borne at the ends of long unbranched pachycaul stems, which are completely covered with large, closely-spaced, spirally-arranged and overlapping leaf-like bracts (Pl. 9, fig. b; Bock, 1969, figs. 528-530, 535; Bock, 1962, figs. 470-471). At least three different types of reproductive structures are here recognized: Bock's species includes several similar and apparently closely related reproductive axes (figures 9a-9c). Fontaine's specific epithet takes priority for at least one of these reproductive axes, while Bock's generic name may have to be retained if his ho1otype cannot be linked to a Fontaine species.
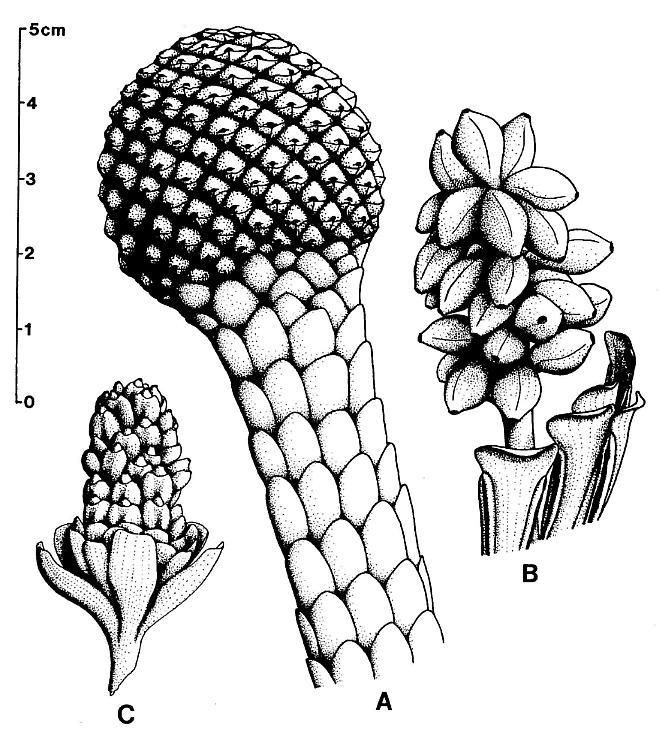
Figure 9. a-c. Zamiostrobus virginiensis Fontaine 1883 or Primaraucaria wielandii Bock 1954 sensu lato. Reconstructions of three different types of fruiting heads and reproductive structures illustrated by Bock (1969) under P. wielandii. -a. TYPE A. -b. TYPE B. -c. TYPE C. See text for further descriptions; scale in cm: x 2.
One of these cone-like structures (Type A) superficially resembles a pineapple in that each spirally-arranged megasporophyll is subtended by a long condup1icate bract that visibly overlaps the apex of the megasporophy11 (Pl. 9, fig. a, b2; figure 9a). Another reproductive axis (Type B) resembles a giant magnolian fruit with subtending 1atrorse laminar stamens (Pl. 9, fig. h, 11s; figure 9b), while a third (Type C) possesses a wide undifferentiated collar of expanded bracts at the base of a long axis bearing megasporophyl1s in the axi1s of large bi1obed bracts (figure 9c; Bock, 1969, fig. 536; Bock, 1962, fig. 470b). All of these reproductive axes resemble the flower-like units terminating secondary axes of Axelrodia burgeri sp. nov., but most have many more carpel-like parts. The perianth-like parts or bracts subtending the solitary megasporophy11s of Axelrodia are present in some but not all of the "Primaraucaria" or "Zamiostrobus" fruiting structures. Even though the megasporophy11 subunits are united to a common axis, they are individually attached by means of elongate pedice1s or secondary branches. They are not borne on tertiary branches as in A. burgeri (Pl. 9, figs. b, d, and h). The long bract-clad axis supporting these fruiting heads may be similar to the slender secondary axes of Axelrodia burgeri sp. nov., but the analogy is uncertain, and a comparison with the long stout primary axes of Axelrodia is equally plausible.
The megasporophy11s of reproductive axis Type B are about 14-16 mm long, solitary, without sub tending bracts, spirally arranged, and pedicellate (Pl. 9, fig. h; figure 9b). Their distally expanding shape, rounded apex, conical stigma-like pore (Pl. 9, fig. i, st), and paired ovules or seeds (Bock, 1969, fig. 545) compare closely with the carpel-like megasporophy11s of Axelrodia. The ovules or seeds are enclosed within a cuticularized ovary wall, the only access to which was through the pore at the conical apex, which may represent the base of a longer deciduous structure. The apparent construction of the megasporophy11 differs significantly from that of the ref1exed "cupu1e" of Caytonia, which has a small lip or opening on one side close to the stalk and ovules that reached maturity with functional micropyles. In addition, the megasporophy11s of "Primaraucaria" Type B were apparently shed at maturity, since they were found by Bock as isolated elements in the sediment (Bock, 1969, fig. 545). Unlike Axelrodia, the megasporophy11 cuticle is glabrous proximally and papillate distally (Pl. 9, fig. i; figure 10h). The basa1ly-narrowing laminar stamen-like microsporophy11s that subtend the reproductive axis are 5-8 mm wide and exceeded 17 mm in length. Each bears two very long anther-Ilke sacs, one on each side. These sacs are covered with a specialized cuticle that is continuous with that of the lamina, and remnants of a possible tapetum were found inside; no pollen was found, since the microsporophy11s had dehisced, and only one chamber was present at maturity (as in many angiosperm anthers).
The megasporophylls of reproductive axis Type A are diamond- shaped in cross section, and arranged in a tight spiral (Pl. 9, figs. a and b; figure 9a) around a non-woody primary axis at the center of numerous decurrent secondary axes (Pl. 9, fig. d). The mass of megasporophylls is ellipsoidal and borne at the apex of a main axis bearing large bracts (Bock, 1969, figs. 534, 539). There is an abrupt transition from megasporophylls to large bracts, with no specialized perianth or pollen-bearing organs. The bracts possess a papillate cuticle with anomocytic stomata. The megasporophylls are wider basally than those of reproductive axis Type B, and contain more seeds. The fruiting head collapsed during fossilization to form a 2 mm thick compression, mainly composed of multiple layers of cuticle and enclosed seeds. The only opening to the megasporophyll is a conical apical pore, visible on surface impressions of the cone-like fruit (Pl. 9, fig. a, st). The apex superficially resembles a cone scale umbo.
A portion of the coalified compression to one specimen (Pl. 9, fig. b, at *) was removed, oxidized, cleared in NaOH, embedded in paraffin, and sectioned. The mounted thin sections showed thick-walled seeds completely surrounded by a pair of cuticles, which were separated by as many as two additional pairs of cu- ticles belonging to surrounding bracts (cf. Pl. 9, fig. d, b2?). Some of the accessory cuticles fuse with the base of the megasporophyll, but are free apically, indicating that they may represent perianth-like parts similar to those around the solitary megasporophylls of Axelrodia. The cuticle of the subtending "conduplicate" bract typically has large epidermal cells with thick cell walls, and can be distinguished from the cuticles of the megasporophyll and adnate accessory cuticles (compare Pl. 9, fig. c with Pl. 9, figs. d-e). One immature and possibly aborted megasporophyll subunit showed an outer bract completely surrounding the collapsed and highly folded cuticles of a megasporophyll, which contained at least four elliptical cuticles of possible ovules in cross section.
The enclosed oval seeds possess a thick granular wall in cross section that strongly stains with safranin O. Isolated seeds, measuring 2.6-2.8 mm in length by 1.6-2.0 mm in width, were removed from dissected megasporophylls; they showed no signs of a micropyle, showing only two shallow pits at one end (Pl. 9, fig. g, arrows). Their walls are typically strongly wrinkled, and there was no pollen found inside the ovary or attached to the seeds. However, small monosulcate pollen was found clinging to the outer distal surface of the megasporophyll. One immature or aborted seed was found with micropyle preserved, and a hilum that lay adjacent to the micropyle (Pl. 9, fig. f, mi and hi); no pol- len was present in the micropyle. No funiculus was preserved and the mature seeds appear to lie loosely within the ovary. Upon dissection of the cuticles, one aborted anatropous ovule, 250 um wide, was discovered still attached by means of a short stalk to the inner cuticle of the megasporophyll (Pl. 9, fig. e).
The absence of pollen within the ovary, and ovules that lost their micropyles during development suggest that pollen germina- tion took place outside the ovary and that ovules did not develop to maturity until fertilized. The similarity of the carpel-like megasporophylls and their sub tending leaf-like parts to those of Axelrodia indicates a fundamental relationship that underlies their superficial differences. The superficial similarity of these cone-like structures to araucarian and cycad cones is eclipsed by major differences in megasporophyll construction, the lack of evidence for woody parts, and the loss of a functional micropyl,e with the development of a thick seed coat.
There is a strong resemblance of reproductive axis Type A to the primitive head of Lesqueria elocata from the mid-Cretaceous(Crane and Dilcher, 1984)- in particular the long series of helically-arranged bracts below the megasporophylls (figures 9a). Both L. elocata and reproductive axis Type C have a collar of expanded bracts below the fruiting structures (cf. figure 9c). Furthermore, the pronounced pedicellate base to the megasporophylls of Axelrodia and "Primaraucaria" may have been retained by the mid-Cretaceous Archaeanthus linnenbergeri (also constricted in Lesqueria, Crane and Dilcher, 1984; Dilcher and Crane, 1984), and the evolution of stigmatic crests along the ventral suture may not have been possible until all enclosing perianth parts were lost (compare Figures 10g-10i). In addition, immature fruiting heads of "Primaraucaria" Bock or "Zamiostrobus" Fontaine resemble the immature flower of Archaeanthus linnenbergeri Dilcher and Crane (compare Bock, 1962, figs. 471b and 471c with Dilcher and Crane, 1984, figs. 10 and 11). The morphological and developmental similarities of these reproductive structures (Types A-C) to Axelrodia, mid-Cretaceous angiosperm flowers, and Recent angiosperms suggest that we may be witnessing some early experiments in the condensation of individual megasporophyll units into a Magnolia-like flower. Dilcher and Kovach (1986) describe Caloda delevoryana from the mid-Cretaceous Dakota Formation, and interpret it as a new unisexual spike-like inflorescence of angiospermous affinity. They interpret the sporophy11s as condup1icate carpels, but as such the lack of perianth parts or their scars make these inflorescences unique among fossil and living angiosperms (Di1cher and Kovach, 1986). Poor preservation of organic remains precluded the recognition of ovules, and the interpretation of the sporophy11s as carpels was based entirely on shape. The diminutive size of the inflorescence is unusual for an ovule-bearing structure, and 2 mm long carpels are rare among primitive living angiosperms. The resemblance of Synangispadixis microsporophy11s to condup1icate carpels of the Drimys or Degeneria type is a similarity that may be important in the interpretation of Caloda. The restriction of sporophy11s to the swollen ends of spira11y-arranged secondary branches is remarkably like that of Synangispadixis, and the development of a pedicel or stipe on the sporophy11s of Caloda is reminiscent of the constricted base to the microsporophy11s of Synangispadixis. The question is raised, therefore, whether C. de1evoryana represents a pollen-producing inflorescence constructed similarly to that of Sanmiguelia, but where the number of microsporophy11s has been greatly reduced. The sporophy11s of Caloda have a ventral suture not unlike the suture of Synangispadixis microsporophy11s, and the unusua11y-wide dorsal vein may instead be the remnants of pollen masses. The enlargements of sporophy11s (Di1cher and Kovach, 1986: figs. 5, 10-13) are unclear about how they attach to the secondary axes, and the illustrations hint that sporophy11 stipes may fuse in pairs before joining the axis. Although the similarity of Caloda and Synangispadixis does not prove that C. de1evoryana is po11iniferous, it does emphasize how much botanists have yet to learn about early angiosperm reproductive morphology, and suggests that the unusual po11iniferous inflorescences of Sanmiguelia may have their counterparts in the Cretaceous.
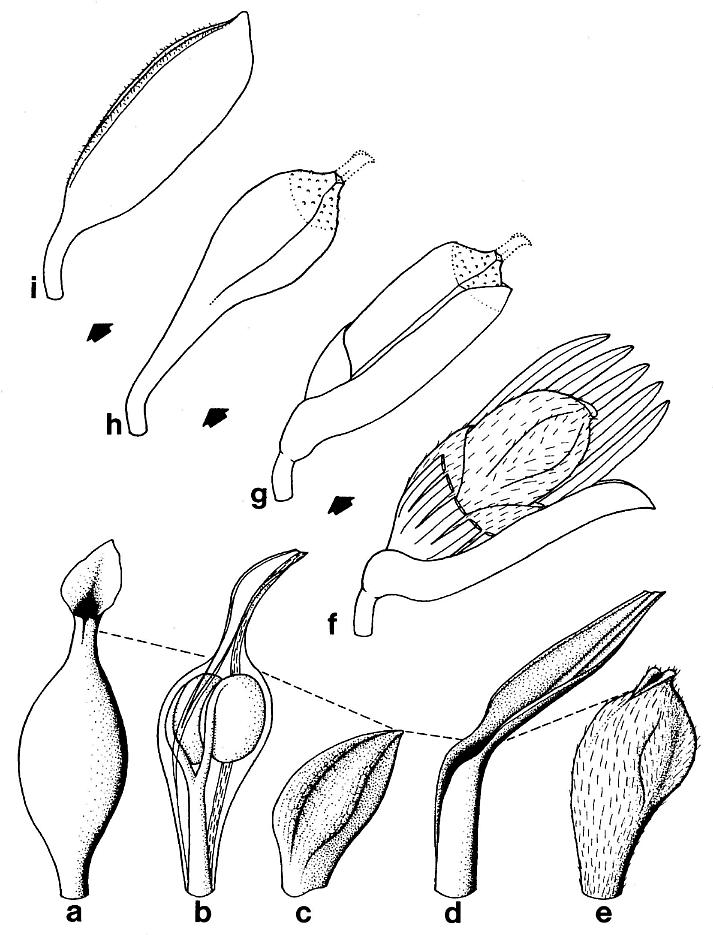
Figure 10. a-e. Comparison of the carpel-like megasporophyll of Axelrodia and the anther-like microsporophyll of Synangispadixis with a small distal three-veined leaf of Sanmiguelia in order to show how the angiosperm carpel and anther may have evolved from the enclosure of an ultimate reproductive axis bearing paired ovules or pollen sacs by the sheathing base to the last leaf; the stigma is correlated with a reduced and specialized leaf blade. f-i. A series of megasporophyll units showing how the simple conduplicate carpel may have evolved from a floral subunit by the loss of surrounding perianth parts and subtending bract. No scale given; actual sizes differ. -a. Zannichellia palustris, Recent monocot (Dahlgren et al., 1985), showing a leaf-like stigma terminating a carpel that resembles a swollen leaf sheath. -b. Hypothetical enclosure of a terminal reproductive shoot by a sheathing leaf base. -c. Biloculate anther-like microsporophyll of S. tidwellii. -d. Third from last leaf on secondary branch, showing a thick central vein flanked by two thin marginal veins. -e. Carpel-like megasporophyll of A. burgeri. -f. Floral subunit or solitary megasporophyll unit of A. burgeri. -g. One of many aggregate megasporophyll subunits from the "Primaraucaria" Type A reproductive axis, Richmond Basin, VA. (Pl. 9, fig. b). -h. One of many aggregate megasporophylls from the "Primaraucaria" Type B reproductive axis, Richmond Basin, VA. (Pl. 9, fig. h). -i. One of many aggregate conduplicate carpel-like megasporophylls from the Archaeanthus linnenbergeri reproductive axis, Dakota Fm., KA. (Dilcher and Crane, 1984).
COMPARISON WITH RECENT PLANTS
The similarity of Sanmiguelia lewisii leaf impressions to the leaves of monocots (Brown, 1956; Tidwell et a1., 1977) has been extended to include venation and cuticular characteristics with the discovery of four orders of parallel venation, several types of cross veins, progressive apical vein fusion, compound primary veins, actinocytic stomata, epidermal trichomes, and evidence for a diffuse intercalary (plate) meristem. Although there is a strong morphological resemblance between the leaves of Sanmiguelia and Veratrum ca1ifornicum (Tidwell et a1., 1977), the .0 poorly organized venation of S. lewisii and the lack of a recognizable pattern of cross veins argue against any close comparison with Recent monocots. Only the seedling leaves of some monocots (e.g. Anthurium 1anceo1atum, Araceae) possess a poorly organized venation that can be compared with that of Sanmiguelia.
The clasping leaf bases, colonial herbaceous habit with an expansive rhizome system, and monocot-1ike inflorescences borne terminally on the main axis above a large spathe-like leaf are further monocot similarities, but the presence of secondary xylem, a primary root system, and an embryo with two cotyledons precludes any close relationship with monocots.
A ring of numerous primary xylem bundles surrounds a large pith (Pl. 2, figs. a-b; Pl. 8, fig. e); based on the impressions of these bundles along the pith casts, they occasionally anast- omosed and bifurcated, creating a loose cylindrical net around the pith. Such an organization is reminiscent of the stem or rhizome anatomy of Ranunculus and Podophyllum (Esau, 1960; personal observation), but Sanmiguelia also had a cambium which produced a significant amount of wood. Unlike Ranunculus, but like many woody dicots, the primary xylem developed centrifugally, with tracheids of increasing diameter outwards (endarch protoxylem).
The presence of secondary xylem produced by a continuous cambium in a plant that otherwise has many similarities with monocots (e.g. Veratrum) is a combination of features unknown in living angiosperms. The vesselless wood compares with that of some primitive dicots, such as Bubbia semecarpoides and Zygogynum vieillardii (Winteraceae), in having numerous high uniseriate rays between large heterocellular multiseriate rays, and tracheids with both scalariform and circular bordered pitting (Dickison, 1975; Eames, 1961). The multiseriate rays allowed multiple leaf traces to supply a parallel-veined leaf. Large multiseriate rays of primitive dicots extend into the secondary body from the parenchymatous gaps, which constitute the interfascicular segments of the primary body (Barghoorn, 1940), as in S. lewisii. Since secondary xylem in most dicots forms a continuous ring of wood mainly after vegetative leaves have fallen off (i.e. vascular traces to leaves are severed), large multiseriate rays in angiosperms may be vestiges of an ancestral condition when clasping leaves persisted during the early stages of wood development. The clusters of multi seriate rays containing leaf traces in the wood of Sanmiguelia may have lead directly to the multilacunar node. If a leaf petiole developed from the system in Sanmiguelia, it could have had the effect of restricting the number of functional wood gaps, leading to trilacunar and unilacunar node arrangements.
The wood preserved in Sanmiguelia roots, however, has tracheids of varying size that range up to vessel-size elements. Although there is little difference in side-wall pitting between the tracheary elements, the end plates of the smaller tracheids are scalariform, while those of the vessel-like elements are porous and possibly simple (Pl. 2, figs. c-e). Furthermore, looking down into the cells in cross section showed scalariform end plates in tracheids, but not in the vessels. The presence of vessels only in the roots compares more with what is known for monocots than for dicots (Dahlgren et al., 1985; Eames, 1961), or the Gnetales (Muhammad and Sattler, 1982). The restricted distribution of vessels in Sanmiguelia could suggest that vessels in the roots of some monocots are either a vestige of the primitive condition or a specialization for a semiaquatic habitat that may have evolved independently many times within the angiosperms and their ancestors.
The apocarpous flowers of the Alismataceae and Zannichelliaceae compare with the flower-like units of Axelrodia burgeri sp. nov. (Dalhgren et al., 1985), but only because they possess terminal unisexual flowers with varying numbers of carpels. If the flower-like units of Axelrodia turn out to have stamen-like organs adaxially attached to their glabrous digitate bracts, the comparison to bisexual apocarpous flowers could be extended to the Annonineae (in the Magnoliales), Aristolochiales, Chloranthales, Laurales, Nymphaeales, Piperales, and Winterales (sensu Walker and Walker, 1984). There are few angiosperms with unicarpellate flowers that can be compared to the flower-like units borne on tertiary branches of Axelrodia. The simple floral subunits of Chloranthus sp. and Lilaea sp. may be homologs of Axelrodia megasporophyll units (cf. Burger, 1977), but if they are, a reduction in the number of perianth parts for Lilaea and Chloranthus is indicated. The flower of Degeneria vitiensis, however, appears to be a reduced terminal flower, because it has a perianth that tends toward trimery, and there is no solitary basal bract as in Axelrodia flower-like units borne on tertiary branches (Dahlgren et al., 1985).
The flowers of the Gnetales resemble the megasporophyll units of Axelrodia. Recent cladistic analyses (Hill and Crane, 1982; Crane, 1985; Doyle and Donoghue, in press) suggest that the Gnetales are the closest living relatives to the angiosperms - an old viewpoint that never completely lost its appeal. The cones of many species of Ephedra contain two ovules, one in the axil of each of the upper bracts. In some species the cones are uniovulate and this condition is commonly the result of the abortion of one ovule and the crowding of the other into a false "terminal" position (Foster and Gifford, 1974). In Gnetum, Ephedra, and Welwitschia each ovule is surrounded by at least two, and sometimes three envelopes, but only the innermost envelope appears to be integumentary (Crane, 1985). If gnetalean flowers are homologs of flower-like units on tertiary branches of Axelrodia, I suggest that two ovules and their surrounding bracteoles (i.e. middle envelope) were enclosed by a third envelope, which became the megasporophyll wall in Axelrodia. The enclosure of two ovules would separate the ontogenies of bracteoles and integuments derived from bracteoles, obscuring the true origin of an outer envelope as in the bitegmic angiosperm ovule. The synangia of Ephedra resemble the secondary branches (microsporangiophores) of Synangispadixis, but any resemblance is overshadowed by the biloculate condition of microsporophylls in both Synangispadixis and angiosperms. Although the resemblance between Axelrodia and gnetalean flowers may reduce the synaptomorphic gap between the angiosperms and the Gnetales, the microsporangiate and megasporangiate organs of Sanmiguelia can no more easily be compared to those of the gnetales than the carpels and anthers of the angiosperms.
The carpel-like megasporophyll of A. burgeri 1) looks morphologically like a primitive angiosperm carpel, 2) developed to a fruiting stage containing seeds as in angiosperms, 3) was demonstrably hollow and contained a pair of anatropous ovules that did not enlarge until late in megasporophyll development (figures 5 and 6), 4) was borne collectively on a recepticle with other carpel-like megasporophylls, and 5) was surrounded by a perianth composed of specialized parts. Although there is no proof that the ovules did not start developing before being fertilized, the absence of a functional or even recognizable micropyle at maturity (assuming that Nemececkigone fabaforma sp. nov. is the dispersed seed of Axelrodia), the anatropous morphology of the enclosed ovules (a feature retained by the developed seed and embryo), and the stigma-like apex on the megasporophyll are a combination of characteristics found together only in angiosperms. The developmental sequence of megasporophyll and ovule strongly indicates that we are dealing with an angiospermous type of carpel for which pollen germinated outside the ovary and the ovule did not develop until after it was fertilized. The large fleshy cotyledons found in one embryo cast (figure 7b) also suggest that significant amounts of food reserve were stored in them, which could indicate an exalbuminous seed, and which could account for the large size of seeds dispersed into an ecoloaically and perhaps climatically unstable red bed environment.
The anther-like microsporophylls of S. tidwellii can be compared only with those of angiosperms, since gymnosperms (including the Gnetales) do not possess microsporophylls that contain two pollen masses, 1) which are separated by a septum that disappears at maturity, 2) which lack developmentally persistent pollen-sac walls, and 3) which are surrounded by endothecial and epidermal wall layers of probable leaf origin (compare figures 3f, 4a-4c, and 10a-10e). In essence, the microsporophyll is angiosporic, i.e. a vessel or container of any kind containing the microsporangia, and as such cannot be distinguished from an angiosperm anther.
The imperforate tectate-granular monosulcate pollen of S. tidwellii (figure 3e), considered by many botanists and palynologists to be the basic pollen type for angiosperms (e.g. Stebbins, 1974; Walker and Walker, 1984; Doyle, 1978), ties in so perfectly with concepts of angiosperm evolution that it makes comparison of Sanmiguelia with any gymnosperm almost pointless. Instead, caution needs to be exercised, because a considerable diversity of reticulate-columellate angiosperm-like pollen is now known from the Late Triassic (Carnian) Dockum Group, TX and Richmond Basin, VA (Cornet, 1977, 1979, 1980, 1981, and in press; Cornet and Olsen, 1985). Not all of these pollen types indicate a monosulcate origin - some are closer in morphology to associated polyplicate pollen, resembling that of Spathiphyllum and Holochlamys of the Araceae, and even the pollen of Ephedra.
Paired sessile anthers borne in clusters on specialized unisexual branches are only known in the Araceae: The synangia of Arisaema atrorubens possess three to five paired anthers on short secondary branches along the basal portion of an elongate unisexual spadix (Dahlgren et al., 1985). The strong resemblanceof these synangia to (shorter versions of) the secondary branches of Synangispadixis tidwellii suggests that A. atrorubens has retained a very primitive type of microsporangiophore, and that the sessile stamen, which is common within the Araceae (French, 1986), is as primitive as the laminar stamen. The morphology of S. tidwellii may represent a specialized adaptation attributable only to Sanmiguelia and its close relatives, as for the spadix of Arisaema.
EVOLUTIONARY SIGNIFICANCE
New and well preserved material representing Sanmiguelia lewisii leaves, stems, roots, and reproductive organs provide conclusive evidence that a plant with taxonomically significant combinations of angiosperm-like characteristics existed during the Late Triassic, over 100 million years before the dicot radiation in the mid-Cretaceous. Some of these characteristics are shared by angiosperms and gymnosperms, and by themselves give little support to an angiospermous affinity - for example, vessel less wood with heterocellular multiseriate and uniseriate rays (Barghoorn, 1940), circular bordered pits (Chamberlain, 1966; Muhammad and Sattler, 1982), leaves with parallel primary venation (cf. Schweitzer, 1977; Andrews, 1967), monosulcate pollen (Hughes, 1976; Crane, 1985), and large se'eds (Andrews, 1967). A score of all characteristics shared with angiosperms, especially the possible synapomorphies, such as megasporophyll and microsporophyll morphology and ontogeny, strongly suggests that Sanmiguelia lewisii (including Axelrodia, Synangispadixis, and Nemececkigone) is more closely related to angiosperms than to any other group of seed plants (Table II).
TABLE II
- Herbaceous semiaquatic growth habit, with clusters of vegetative axes interconnected by a subsoil rhizome system, and satellite axes connected to clusters by very long unbranched(?) rhizomes.
- Large monocot-like vegetative leaves with clasping to sheathing bases, and numerous plications that parallel major venation in blade.
- Secondary branches borne in the axils of leaves, and bearing a decreasing series of parallel-veined leaves down to small terminal leaves with only three veins.
- Intercalary meristems in the leaves and megasporophylls, inferred from venation patterns and changes in morphology with growth.
- Tertiary and quaternary cross veins, and apical vein fusion in the leaves; apical leaf meristems that were sensitive to damage, sometimes leaving leaf apex incomplete.
- Primary and secondary veins that are composed of bundles of smaller veins, and higher rank parallel veins that form elongate loops with their vein of origin.
- Epidermal hairs and dendroid trichomes on the leaves; long multicellular and glandular hairs on the megasporophylls and protective bracts.
- Crowded actinocytic stomata with variable numbers of subsidiary cells; most oriented transversely to the axis of the leaf.
- Very tall uniseriate and large heterocellular multiseriate rays in wood; secondary tracheids with helical-scalariform pitting, which may be more common in early wood; circular bordered pitting more common in late wood (to be presented in detail elsewhere).
- Vessels in the roots, but not in the stems; crowded simple pits on the side walls of tracheids and vessels in roots; scalariform end plates on tracheids, but porous end plates on vessels. in the roots (to be presented in more detail elsewhere).
- A large central pith containing remnants of possible medullary vascular bundles; pith surrounded by a ring of numerous anastomosing and bifurcating protoxylem bundles; protoxylem and metaxylem elements developing centrifugally, and composed mainly of tracheids with scalariform and helical pitting (to be presented in detail elsewhere).
- Large panicle-like male inflorescence probably subtended by a large spathe-like leaf; larger panicle-like female (unisexual?) inflorescence with clasping bracts and sheathing cataphylls sub tended by a large spathe-like leaf.
- Sessile paired biloculate microsporophylls, anther-like, borne on fleshy axes alonl naked main axis of male inflorescence.
- Separate megasporophylls, carpel-like, with a short pedicellate base, apically-expanding unsutured conical body, stigma-like apex, and a pair of ovules attached parietally within an ovary; megasporophyll(s) surrounded by leaf-like perianth parts; significant size difference between immature and mature megasporophyll.
- Anatropous ovules that show a delayed development to a symmetrical (mirror-image) pair of large seeds only after megasp6rophyll has reached about 40% of its maximum size, indicating probable post-fertilization development.
- Dispersed seeds bean-shaped, possessing one very convex side and one slightly convex to flat side, a hard smooth seed coat, and micropylar pit adjacent to hilum scar; embryo cast with two fleshy cotyledons, one of which is distinctly smaller than the other; both attached to distal end of an elongate hypocotyl with plumule between cotyledons at one end and radicle at other end adjacent to micropylar pit.
- Simple tectate-granular psilate monosulcate pollen with a poorly developed and irregular sulcus.
No angiosperm presently described from the Cretaceous is as well known as Sanmiguelia sensu lato. Whereas previously described leaf impressions of Sanmiguelia provide insufficient information to resolve its systematic affinities, the interpretations here based on currently available data on reproductive morphology clearly establishes it as related to angiosperms. Although further information on the gametophyte, fertilization, etc. would be helpful, those features are rarely preserved in fossil material. The megasporophylls (Axelrodia) and microsporophylls (Synangispadixis) so closely resemble carpels and anthers, respectively, that they should probably be called by those terms; any concern for an implied homology by the use of angiospermous terminology is important only if the large number and comprehensive distri- bution of angiosperm-like characteristics in Sanmiguelia is considered a remarkable example of convergent evolution in an extinct group of seed plants not related to angiosperms. That interpretation, however, in not adopted here. Whereas previous claims of pre-Cretaceous angiosperms could be dismissed because of inadequate preservation or evidence, any interpretation of Sanmiguelia is subject to critical examination of the specimens. What is now known about Sanmiguelia reveals more about possible angiosperm ancestry than any described Cretaceous fossil, and helps explain why pre-Cretaceous angiosperms are so rare.
Axelrodia burgeri sp. nov., the female inflorescence of Sanmiguelia lewisii, preserves a transitional stage in the evol- ution of a terminal flower through the aggregation, perhaps by condensation, of homologous parts (cf. Burger, 1977, 1980). The solitary flower-like units borne on tertiary branches resemble gnetalean flowers and cordaitean fertile short shoots, whose scale leaves developed clasping or sheathing bases, causing them to become tightly arranged in spirals or whorls in the axil of a subtending elongate bract (cf. Florin, 1951).
The evolution of the carpel may have started with the re- traction of an ovuliferous short shoot (cf. progenesis), causing its ovules to be encircled by the sheathing base of an enlarged sub tending scale leaf. A similar retraction of the gynoecium below the level of the perianth is a common concept used to visualize the origin of epigyny. The resulting carpel (figure lOb) would have resembled a cupule, but with a narrow distal opening that extended as a suture along the ventral margins down to the point where the sheathing base formed a tube. Zannichellia palustris, a Recent monocot, possesses carpels that conceptually resemble an early stage (figure lOa). The key to the evolution of the carpel, therefore, may have been the three-veined sheathing leaf. This feature is significantly absent in the Gnetales, considered by some to be a sister group to the angiosperms (Hill and Crane, 1982; Crane, 1985).
Once the sheathing leaf base had "swallowed" the ovuliferous branch, it would have been constricted above the level of the ovules and had a limited capacity for enlargement without an in- tercalary meristem. Since mature carpels of A. burgeri are many times the size of immature carpels, and they developed shoulder pockets that "anticipated" the enlargement of the enclosed seeds, an intercalary meristem is indicated. Meristematic activity is also indicated for the vegetative leaf, as would be expected if carpels and leaves are homologs. An intercalary meristem probab- ly developed before a conduplicate or sheathing leaf enclosed a terminal ovuliferous branch, simply because of the changes required in leaf size and shape during ovule or seed development. By itself, the development of a sheathing base requires that the leaf expand as the enclosed stem widens. Even if initial ovule size was small, like that of Caytonia (Harris, 1932) or Glossopteris (Gould and Delevoryas, 1977), delayed ovule development, as in Axelrodia, until after fertilization would have ruptured any carpel wall that did not expand internally with ovule or seed growth.
The free distal portion of the leaf blade may initially have served as a catching device for pollen, supplementing any micropylar extensions. Pollen germination on integumentary appendages or cone scales is known for some conifers (Doyle, 1945). Drops of moisture at the leaf tip may have caught pollen and transported it to the ovary as the drops ran down the leaf blade. Some of the pollen may have germinated inside the ovary adjacent to but outside micropylar chambers. As selection favored fertilization from pollen in the ovary, ovules became anatropous and basally attached, perhaps because the pollen, lacking bladders, tended to sink to the base of the ovary.
Coincidentally, secretory hydathodes may have evolved at the leaf apex (Melville, 1962), exudates of which facilitated pollen capture and transportation in fluid drops. As the drops ran down the axis of the leaf to the ovules, they supplemented the function of pollen drops at the ends of one or more micropylar tubes; selection increased the amounts of nutrient in the fluid, allowing pollen tubes to grow increasing distances to the ovules. Eventually, a specialized mucilage-coated transmission tissue evolved from the leaf apex down into the ovary, and the ovules evolved strong pollen-tube attractants that produced a chemical gradient in the fluids and mucilage coating the ovary wall.
The genetic suppression of the ovule-bearing axis may have extended to the ovules, thereby setting the stage for post-fertilization growth and endosperm development. As the ovule-bearing axis became suppressed, ovules attached to the ventral or adaxial side of the sheathing leaf base, because that is the side closest to the axis, and because a wide central vein ran down the decurrent dorsal or abaxial side. The leaf apex became reduced in size as transmission tissue became more effective (figure 10e), but it retained the venation of a leaf in a condensed form because of the specialized needs of the glandular stigma (figure 7a; cf. Melville, 1962). The bilobed morphology of the stigma (an angiosperm characteristic) may have resulted from the premature loss of the apical meristem, a typical characteristic of Sanmiguelia leaves. The anthers developed in a similar manner as the carpels, but because the leaf blade was superfluous and non-functional, it was completely lost (figure 10c).
The conduplicate carpel has long been considered the primitive form within the Ranales, and the basis for carpel evolution in the angiosper8s (Bailey and Swamy, 1951). The concept arose out of the similarity of the carpel of Drimys piperita (Winteraceae) to a folded leaf with three veins. The primary distinction between the conduplicate theory of carpel evolution and the sheathing leaf base theory presented above is whether a leaf blade joined with and wrapped around an ultimate ovuliferous branch, or whether the ovuliferous branch contracted into the basal tube of a sheathing leaf. However, the conduplicate theory assumes that a pollen-tube transmission tissue evolved after micropylar extensions were lost and after the sides of the leaf came together, and that ovules were initially attached near the margins of the leaf (as in some Paleozoic pteridosperms and cycads; Doyle and Donoghue, in press). Relatively few examples are known outside the angiosperms where pollen germinates on non-integumentary organs; in Araucaria (Doyle, 1945), and perhaps also in some Mesozoic Cheirolepidaceae, which have megasporophylls of similar construction, the pollen germinates on the ovuliferous scale. Once the conduplicate leaf was closed, there would be no dual mechanism of bringing the pollen near the egg as with the sheathing leaf base theory, which allows for the gradual evolution of a pollen-tube transmission tissue and the loss of a micropylar tube. The megasporophyll of Glossopteris folded and enclosed small ovules in accordance with the conduplicate theory, but specialized micropyles communicated with the opening along the suture, and the ovary became filled with a cancellous tissue (Gould and Delevoryas, 1977).
Angiospermous fruiting heads from the Richmond Basin of Virginia (see figure 9, and comparison with fossil plants above) possess pedicellate carpels similar to those of Axelrodia and Archaeanthus Dilcher and Crane (1984), but the stigmatic regions of those cone-like fruits are restricted to the apical or distal ends of the carpels, and form no part of the ventral or adaxial surface. Ventral paired stigmatic crest may not have evolved until after enclosing perianth parts were lost and the number of paired ovules greatly increased along the ventral side of the carpel (figures 10f-10i). The condensation of many megasporo- phyll subunits into a strobilus-like flower, a sequential loss of perianth parts around each floral subunit (figures 10f-10i), and the evolution of laminar stamens which sub tend the carpels on the same axis (Pl. 9, fig. h) may have lead to an early evolutionary specialization within the angiosperms, whose descendants are grouped today in the Magnoliales. Perhaps that is why the Drimys-type conduplicate carpel is unknown for the monocots, and why the apocarpous carpel with an apical stigma is found in Sanmiguelia. Burger (1980) anticipated the discoveries presented here in his analysis of angiosperm floral evolution:
"Unfortunately, a strobilus-like flower in an ancient and relictual order is no guarantee that this kind of flower is the single ancestral type from which all other contemporary flowers descended. The flowers of the Magnoliaceae may represent some early experiments in floral organization; they need not have been the archetype from which all other flowers have evolved. The diversity of floral organization within the Magnoliales and the closely related Laurales suggests that they include a variety of basic floral plans, ranging from the long floral receptacle of the Magnoliaceae and the cupulate receptacles so common in the Laurales to the very simple flowers of the Chloranthaceae. This diversity of floral architecture within the Magnoliales and related orders supports the idea that contemporary flowers may be polyphyletic; that is, made up of homologous parts that have come together to form flowers in different ways in different lineages. There is a variety of evidence supporting this point of view. Both floral ontogeny, floral structure, and chemistry are very distinctive in the Magnoliales, implying that they are an early side branch of angiosperm evolution rather than a basic stem group" (Burger, 1980, p. 1).
REASONS FOR A POOR PRE-CRETACEOUS ANGIOSPERM RECORD
Axelrod (1952) originally proposed a theory that angiosperm history extended back to the Permo-Triassic, perhaps to pre-magnolian proangiosperms, and that an upland origin could explain their poor pre-Cretaceous fossil record. Scott, Barghoorn, and Leopold (1960) countered by stating that all records of pre-Cretaceous angiosperms are suspect, and that angiosperms arose in the Middle Mesozoic. Axelrod (1961) rebutted their ideas, and later extended his theory (Axelrod, 1970) to include the effects of ocean-floor spreading on early angiosperm history, interpreting the transtropical distribution of many angiosperm families as indicating a pre-Cretaceous origin. Doyle (1977; 1978) and Hickey (Hickey and Doyle, 1977) followed Scott, Barghoorn, and Leopold's lead with a detailed study of patterns of evolution of angiosperms in the Early Cretaceous, concluding that a signifiicant pre-Cretaceous history for angiosperms does not exist, and that the earliest Cretaceous angiosperm record gives us an ap- proximate time of angiosperm origin.
The scarcity of pre-Cretaceous angiosperm fossils can be put into perspective, now that we have several probable examples from the Triassic for comparison. Sanmiguelia would not be known were it not for the unusual mode of in situ preservation. Its leaves and reproductive organs were too fragile or large to be transported any siinificant distance without major damage. Some of its leaves were so large that only the best preservation (i.e. major portions of leaves with preserved cuticles and veins) would reveal their identity. Yet, this plant grew in environments whose litho-facies have yielded common well-preserved fronds of ferns and cycadeoids (Ash, 1969; 1972a; 1972b; 1974; 1975; 1978), and its growth habit suggests that it may have been a" common waterside element in the tropical floodplain flora of the Late Triassic Dockum Group (cf. Cornet and Olsen, 1985). The pollen, although produced in large quantities, comprises less than one percent of all palynoflorules examined, and cannot be readily distinguished from that of gymnosperms.
The Magnolia-like reproductive axes ("Primaraucaria": Bock, 1954; 1969) found in the Richmond Basin Triassic of Virginia owe their preservation to lowland coal-swamp deposits. Their sig- nificance went unrecognized, not because of poor preservation, but because of their superficial resemblance to large coniferalean and cycadalean cones (figure 9a-9c). Similarly, the fruiting heads of Lesqueria elocata from the mid-Cretaceous were originally mistaken for bennettitalean flowers (Crane and Dilcher, 1985). The small monosulcate pollen found clinging to the outside of carpel cuticles of "Primaraucaria", if it belongs to these plants, is indistinguishable from monosulcate bennettitalean pollen in the associated sediments.
CONCLUSIONS
The leaves, stems, roots, and growth habit of Sanmiguelia lewisii are now known in sufficient detail to make the first reliable evaluation of the systematic relationships of this plant. Associated reproductive axes, some of which were found in organic connection with or overlapping Sanmiguelia leaves and stems, indicate predominantly unisexual inflorescences with distinctly angiospermous characteristics.
The overall characteristics of Sanmiguelia lewisii and its reproductive structures indicate a dicotyledonous plant which can be assigned to neither the Dicotyledoneae nor the Monocotyledoneae sensu stricto, because it possessed combinations of characteristics that are no longer found in either subclass (such as monocot-type leaves borne on stems with a dicotyledonous type of secondary xylem), and possessed characteristics that are no longer found among living angiosperms (such as clusters of floral subunits borne separately on tertiary branches).
Both types of inflorescences possess characteristics that compare more favorably with monocots than dicots, particularly the carpels with apical stigmas in A. burgeri, the synangia-like secondary axes and sessile anthers of S. tidwellii, the long primary axis bearing clasping bracts that change to sheathing cataphylls distally in A. burgeri, and the large spathe-like leaf that probably subtended both types of panicle-like inflorescences. The herbaceous habit of S. lewisii is found in both monocots and dicots, but a primary tap-root is characteristic of only the dicots. The presence of two cotyledons is obviously a dicotyledonous characteristic, and the asymmetrical morphology of the cotyledons with one being significantly smaller than the other is found in some herbaceous Ranales, such as Ranunculus (Eames, 1961).
The distinctly monocotyledonous leaf morphology and leaf venation, combined with monocot-like inflorescences and semi-aquatic habitat, suggest that Sanmiguelia may have been a primitive dicot below the level of extant Magnoliidae and close to the evolutionary branch between monocots and dicots. This does not necessarily mean that many of its monocot characteristics are derived (cf. Burger, 1981); S. lewisii may have been close to the morphology of an angiosperm ancestor, because its sheathing leaves with parallel veins, apical vein fusion, and intercalary meristems may have been necessary for the evolution of the angiosperm carpel.
The presence of strobilus-like angiospermous fruits resem- bling large magnolian flowers in similar age deposits of Virginia suggests that major innovations in floral structure were evolving in the Late Triassic. The occurrence of diverse types of reticulate-columellate angiosperm-like pollen in those deposits, including monosulcate, trisulcate, zonasulculate, pseudotricolpate, heterocolpate, and spiraperturate types, some of which strongly resembles monocot pollen (e.g. Liliacidites: Cornet, 1977, 1979, 1980, 1981, in press), supports the megafossil evidence by suggesting a pattern of evolutionary history for flowering plants similar to that of mammals, with an early Mesozoic origin followed by low diversity and a delayed dicot radiation (Crane, 1985).
LITERATURE CITED
Arnold, C.A., 1963, Cordaites-type foliage associated with palm~ like plants from the Upper Triassic of southwestern Colorado, Maheshwari Commem. vol., J. Ind. Bot. Soc., V. 42-A, p. 1-4.
Ash, S.R., 1969, Ferns from the Chinle Formation (Upper Triassic) in the Fort Wingate Area, New Mexico, Geol. Surv. Prof. Pap. 613-D, 52 pp.
-,- 1972a, Upper Triassic Dockum flora of eastern New Mexico and Texas, New Mexico Geol. Soc. Guidebook, 23rd Field Conf., p. 124-128.
-,- 1972b, Late Triassic plants from the Chinle Formation in north-eastern Arizona, Palaeont., V. 15, Part 4, p. 598-618.
-,- 1974, Upper Triassic plants of Canon del Cobre, New Mexico, New Mexico Geol. Soc. Guidebood, 25th Field Conf., p. 179-184.
-,- 1975, The Chinle (Upper Triassic) flora of south-eastern Utah, Four corners Geo1 Soc. Guidebook, 8th Field Conf., p. 143-147.
-,- 1976, Occurrence of the controversial plant fossil Sanmiguelia in the Upper Triassic of Texas, Jour. Pa1eont., V. 50, No.5, p. 799-804.
-,- 1978, Stratigraphy of the Ciniza Lake Beds and related strata, in Geology, paleontology, and paleoecology of a Late Triassic lake, western New Mexico, S. R. Ash, editor, Brigham Young Univ. Geo1. Studies, V. 25, Part 2, p. 1-14.
Axelrod, D.I., 1952, A theory of angiosperm evolution, Evolution, V. 6, No.1, p. 29-60.
-,- 1961, How old are the angiosperms?, Amer. Jour. Sci., V. 259, p. 447-459.
-,- 1970, Mesozoic paleogeography and early angiosperm history, Bot. Rev., V. 36, No.3, p. 277-319.
Bailey, I.W. and B.G.L. Swamy, 1951, The condup1icate carpel of dicotyledons and its trends of specialization, Amer. Jour. Bot., V. 38, p. 373-379.
Barghoorn, E.S. Jr., 1940, The ontogenetic development and phylogenetic specialization of rays in the xylem of dicotyledons. I. The primitive ray structure, Amer. J. Bot., V. 27, p. 918-928.
Becker, H.F., 1964, Paleobotanical exploits in Colorado and Kansas, Garden J. (N.Y.B.G.), V. 14, No.6, p. 231-233.
-,- 1972, Sanmiguelia, an enigma compounded, Pa1aeont. Abt. B, V. 138, p. 181-185.
Bock, W., 1954, Primaraucaria, a new araucarian genus from the Virginia Triassic, J. Pal., V. 28, p. 32-42.
-,- 1962, Systematics of dichotomy and evolution, Geo1. Cntr. Res. Ser., North Wales, PA, V. 2, 300 pp.
-,- 1969, The American Triassic flora and global distribution, Geo1. Cntr. Res. Ser., North Wales, PA, Vs. 2-3, 406 pp.
Brown, R.W., 1956, Palm-like plant s from the Dolores Formation (Triassic), southwestern Colorado, U.S. Geol. Surv. Prof. Pap. 274-H, p. 205-209.
Burger. W.C., 1977, The Pipera1es and the monocots. Alternate hypotheses for the origin of monocotyledonous flowers, Bot. Rev., V. 43, No.3, p. 345-393.
-,- 1980, On the origin' of flowers, IAAP Newsletter, V. 6, No.1, p. 1-2.
-,- 1981, Heresy Revived: The monocot theory of angiosperm origin, Evol. Theory, V. 5, p. 189-225.
Chamberlain, C.J., 1966, Gymnosperms, structure and evolution, Dover Pub., Inc., NY, 484 pp.
Cornet, B., 1977, The palynostratigraphy and age of the Newark Supergroup, unpublished Ph.D. thesis, The Pennsylvania State University, 505 pp.
-,- 1979, Angiosperm-like pollen with tectate-columellate wall structure from the Upper Triassic (and Jurassic) of the Newark Supergroup, U.S.A., Palynology, V. 3, Abstr., p. 281-282.
-,- 1980, Tropical Late Triassic monosulcate and polysulcate angiospermid pollen and their morphological relationship with associated auriculate polyplicate pollen, 5th International Palynological Conference, Cambridge 1980, Abstr., p.91.
-,- 1981, Recognition of pre-Cretaceous angiosperm pollen and its relationship to fossil polyplicate pollen, Palynology, V. 5, Abstr., p. 212-213.
-,- in preparation, Triassic and Jurassic pollen with angiospermous affinites, A.A.S.P. Contribution Series, 101 pp. [this paper was never published; instead, Cornet, B., Late Triassic angiosperm-like pollen from the Richmond rift basin of Virginia, U.S.A., Palaeontographica, Abt. B, 213: p. 37-87.]
-,- and P.E. Olsen, 1985, A summary of the biostratigraphy of the Newark Supergroup of eastern North America with comments on Early Mesozoic provinciality, in III Congreso Latinoamericano de Paleontologia. Mexico, R. Weber, editor, Simposio sobre Floras del Triassico Tardio, su Fitogeografia y Paleoecologia. Memoria, p.67-81.
Crane, P.R., 1985, Phylogenetic analysis of seed plants and the origin of angiosperms, Ann. Missouri Bot. Gard., V. 72, No.4, p. 716-793.
-,- and D.L. Dilcher, 1984, Lesqueria: an early angiosperm fruiting axis from the mid-Cretaceous, Ann. Missouri Bot. Gard., V. 71, No.2, p. 384-402.
Dahlgren, R.M.T.; H.T. Clifford, and P.F. Yeo, 1985, The families of the monocotyledons, structure, evolution, and taxonomy, Springer-Verlag, NY, 520 pp.
Dickison, W.C., 1975, The bases of angiosperm phylogeny: vege- tative anatomy, Ann. Missouri Bot. Gard., V. 62, p. 590-620.
Dilcher, D.L. and P.R. Crane, 1984, Archaeanthus: an early angiosperm from the Cenomanian of the western interior of North America, Ann. Missouri Bot. Gard., V. 71, No.2, p. 351-383.
-,- and W.L. Kovach, 1986, Early angiosperm reproduction: Caloda delevoryana gen. et sp. nov., a new fructification from the Dakota formation (Cenomanian) of Kansas, Amer. J. Bot., V. 73, No.8, p. 1230-1237.
Doyle, J.A., 1973, Fossil evidence on early evolution of the monocotyledons, in The monocotyledons: their evolution and comparative biology, Quart. Rev. BioI., V. 48, No.3, p. 399-413.
-,- 1977, Patterns of evolution in early angiosperms, in Patterns of evolution, A. Hallam, editor, Elsevier Sci. Publ. Co., Amsterdam, p. 501-546.
-,- 1978, Origin of angiosperms, Ann. Rev. Ecol. Syst., V. 9, p. 365-392.
-,- and L.J. Hickey, 1976, Pollen and leaves from the mid-Cretaceous Potomac Group and their bearing on early angiosperm evolution, in Origin and early evolution of angiosperms, C.B. Beck, editor, Columbia University Press, NY, p. 139-206.
-,- and M.J. Donoghue, in press, The origin of angiosperms: A cladistic approach, in The origin of angiosperms and the biological consequences, E.M. Friis, W.G. Chaloner, and P.R. Crane, editors, Cambridge Univ. Press, Cambridge, 25 pp.
Doyle, J., 1945, Developmental lines in pollination mechanisms in the coniferales, Scient. Proc. R.D.S., V. 24, No.5, p. 43-62.
Dunay, R.E., and M.J. Fisher, 1979, Palynology of the Dockum Group (Upper Triassic), Texas, U.S.A., Rev. Palaeobot. Palynol., V. 28, p. 61-92.
Eames, A.J., 1961, Morphology of the angiosperms, McGraw-Hill Book Co., NY, 518 pp.
Esau, K., 1960, Anatomy of seed plants, John Wiley & Sons, Inc., NY and London, 376 pp.
Florin, R., 1951, Evolution in cordaites and conifers, Acta Horti Bergiani, V. 15, No. 11, p. 285-389.
Fontaine, W.M., 1883, Contributions to the knowledge of the older Mesozoic flora of Virginia, U.S. Geol. Surv. Monographs, V. 6, 144 pp.
Foster, A.S. and E.M. Gifford, Jr., 1974, Comparative morphology of vascular plants, W.H. Freeman and Company, San Francisco, 751 pp.
French, J.C., 1986, Patterns of stamen vasculature in the Araceae, Amer. J. Bot., V. 73, p. 434-449.
Gould, R.E. and T. Delevoryas, 1977, The biology of Glossopteris: evidence from petrified seed-bearing and pollen-bearing organs, Alcheringa, V. 1, p. 387-399.
Harris, T.M., 1932, The fossil flora of Scoresby Sound East Greenland, Part 3: Caytoniales and Bennetitales, Meddelelser om Gronland, V. 85, No.5, 133 pp.
Hickey, L.J. and J.A. Doyle, 1977, Early Cretaceous fossil evidence for angiosperm evolution, Bot. Rev., V. 43, p. 2-104.
Hill, C.R. and P.R. Crane, 1982, Evolutionary cladistics and the, origin of angiosperms, in Problems of phylogenetic reconstruction, K.A. Joysey and A.E. Friday, editors, Syst. Assoc. Spec. Vol., Academic Press, London and NY, No. 21, p. 269-361.
Hughes, N.F., 1976, Palaeobiology of angiosperm origins, Cambridge Univ. Press, Cambridge, 242 pp.
Leschik, G., 1955, II. Die Iso-und mikrosporen, in Krausel, R., and Leschik, G., Die Keuperflora von Neuewelt bei Basel, Schweizerische Palaeontologische Abhandlungen, Memoires Suisses de Paleontologie, V. 72, p. 5-68.
Mädler, K., 1964, Die geologische verbreitung von sporen und pollen in der deutschen Trias, Beihefte zum Geologischen Jahrbuch, V. 65, 185 pp.
Melville, R., 1962-1963, A new theory of the angiosperm flower: I, Kew Bull., Royal Bot. Gard., V. 16, No.1, pp. 1-50.
Muhammad, A.F. and R. Sattler, 1982, Vessel structure of Gnetum and the origin of angiosperms, Amer. J. Bot., V. 69, No.6, p. 1004-1021.
Niklas, K., 1981, Airflow patterns around some early seed plant ovules and cupules: Implications concerning efficiency in wind pollination, Amer. J. Bot., V. 68, No.5, p. 635-650.
Olsen, P.E., 1984, Comparative paleolimnology of the Newark Supergroup: a study of ecosystem evolution, unpubl. Ph.D. thesis, Yale University, Vs. 1 and 2, 726 pp.
Schweitzer, H.-J., 1977, Die Rato-Jurassischen floren des Iran und Afghanistans, Palaeontographica, Abt. B, V. 161, p. 98-145.
Stebbins, G.L., 1974, Flowering plants, evolution above the species level, The Belknap Press of Harvard Univ. Press, Cambridge, MA, 399 pp.
Tidwell, W.D.; A.D. Simper, and G.F. Thayn, 1977, Additional information concerning the controversial Triassic plant: Sanmiguelia, Palaeont. Abt. B, V. 163, p. 143-151.
Walker, J.W. and A.G. Walker, 1984, Ultrastructure of Lower Cretaceous angiosperm pollen and the origin and early evolution of flowering plants, Ann. Missouri Bot. Gard., V. 71, No.2, p. 464-521.
PHOTOGRAPHIC PLATES
Plate 1, figs. a-h. Sanmiguelia lewisii Brown. -a. PP34363. Fallen axis with two small leaves attached, showing sheathing leaf base (sIb). -b. Enlargement of fig. a showing leaf plications, torn leaf apex, and free part to sheathing leaf base (s1b). -c. PP34359. Folded distal portion of a large vegetative leaf, showing well-preserved parallel venation and elongate resin bodies (arrows). -d. SLIDE-118. Acetate transfer of leaf showing elongate resin body over a vein; x 81. -e. SLIDE-118. Acetate transfer of leaf showing annular-helical tracheids of a quaternary vein; x 370. -f. Lower half: Helical-scalariform tracheids macerated from inner part of secondary xylem; x 300; Upper half: THIN SECTION-122. Helical-scalariform tracheid in a silicified, protoxylem bundle of inflorescence axis to S. tidwellii for comparison; x 450. -g. Acetate transfer of basal portion of leaf 4', showing bifurcating secondary veins within a dividing primary vein; portions of coalified veins removed during transfer, leaving white areas and underlying cuticle exposed; x 20. -h. Acetate transfer of basal portion of leaf showing secondary veins diverging laterally and interconnected by cross veins (arrows); x 20.