EVOLUTIONARY TRENDS: IN PLANTS. VOL. 3(1) 1989
THE REPRODUCTIVE MORPHOLOGY AND BIOLOGY OF SANMIGUELIA LEWISII, AND ITS BEARING ON ANGIOSPERM EVOLUTION IN THE LATE TRIASSIC
BRUCE CORNET
Lamont-Doherty Geological Observatory of Columbia University, Palisades, New York 10964,
USA
Key words
Sanmiguelia - Angiosperm - Late Triassic - Flowers - Origin
Abstract
The discovery of new reproductive material, including isolated flowers and carpels of Axelrodia burgeri Cornet (the female inflorescence of Sanmiguelia lewisii Brown) at the Sunday Canyon locality in Texas, provides documentation for an apocarpous angiosperm-like flower with closed carpels and a differentiated perianth. An isolated 14 mm long carpel compression was dissected and analyzed. It possessed an outer thick cuticle with numerous hair bases and scattered stomata, an inner thin ovary wall cuticle with abundant stomata, a pair of small (1 mm) basal ovules, one positioned on either side of a closed ventral suture, and a pair of scale-like organs (adnate to the ovary wall and possessing pollen-tube transmission-tissue characteristics) that flanked the ventral suture and connected stigma with ovules. Resin-like beads reside within the carpel wall, and two or three large glands' are positioned near an apical stigma. Evidence from a silicified anatropous ovule and ovule compression suggests the presence of two integuments. The development of carpel and ovules/seeds is graphed and compared to patterns of comparable organ development for Magnolia spp., a monocot, and an outgroup conifer. Axelrodia and the angiosperms all show, in contrast to the conifer an initial delay in ovule growth until the carpels reach a similar relative size on the growth curve, indicating that fertilization was probably required in Sanmiguelia before the ovules continued development, an interpretation supported by the morphology of mature seeds with embryo casts. Solitary male flower-like units of Synangispadixis tidwelli Cornet (the male inflorescence of Sanmiguelia) and female flower-like units of Axelrodia are homologized with male and female flower-like units belonging to unisexual inflorescences of an associated gnetalean-like plant, reducing the morphological gap between the Gnetales and Angiospermae. The male flower-like units of Sanmiguelia, which bear hundreds of spirally-arranged paired biloculate anthers on the outside of a tubular or false axis, are homologized with the carpels of Axelrodia, providing support for the classical theory that the angiosperm laminar stamen is in fact a carpel homologue, but that a single pair of anthers is the product of reduction. The variable position of angiosperm anthers on the stamen is attributed to a primitive cylindrical and fleshy filament as in Chloranthus. Direct and indirect evidence is presented for pollination by phytophagous insects. Sanmiguelia is interpreted as a very primitive angiosperm (i.e. taxonomically above the level of Class) that combined both monocot and dicot characters.
Introduction
For a period of thirty years published descriptions of Sanmiguelia lewisii Brown remained at a coarse vegetative level (Brown, 1956; Arnold, 1963; Bock, 1969; Ash, 1976; Tidwell et al., 1977) with little information on reproductive structures except possible associated seeds (Becker, 1972). However, the discovery of a colony of Sanmiguelia preserved in growth position with associated well-preserved reproductive structures in northwestern Texas changed the nature of the controversy about this Late Triassic plant (Cornet, 1986; Crane, 1988). For the first time paleobotanists could focus on critical vegetative, anatomical, and reproductive characters in order to assess its taxonomic affinities and phylogenetic position. Crane (1988: p. 779), in his review of Cornet (1986), states, 'Although [he] provides bold interpretations of these features, the evidence is not uniformly strong [the reproductive organs] are obviously complex structures but have so far yielded very few unequivocal points of reference from which their structure can be interpreted with reasonable certainty.... and better preserved material may be needed to adequately test Cornet's initial interpretations.' This paper describes or references all of the known reproductive material, including new specimens, some of which are more complete and better perserved than those available to Cornet (1986). Critical aspects of Sanmiguelia's morphology pertaining to reproductive biology and affinity are addressed here. The author suggests that Cornet (1986) be read for a more complete understanding of Sanmiguelia's vegetative morphology and anatomy, and for a justification of terminology.
S. lewisii was a semiaquatic woody herbaceous plant that resembled extant Veratrum (Liliaceae) in form and stature, but which produced a series of clustered innovations of a few years' duration along a spreading rhizome (Cornet, 1986). Its rhizomatous habit, vesselless secondary xylem of restricted development in the lower part of the stem, vessels in the secondary xylem of roots, and a dicotyledonous embryo (Cornet, 1986) are characters shared with extant Sarcandra (Chloranthaceae: Carlquist, 1987). Its large plicate leaves had clasping or sheathing bases, four orders of parallel venation, cross veins, and apical vein fusion, giving them a distinctive monocot-like construction. Its unisexual reproductive structures were dimorphic as in Hedyosmum (Chloranthaceae: Endress, 1987). The male inflorescence was a simple spike, while the female inflorescence was a large panicle (Cornet, 1986) that resembled the inflorescence of extant Yucca with its elongate basal axis and apical compound branching. Large apocarpous flowers with an elaborate perianth were borne by the female inflorescence (Cornet, 1986), while the male flowers were simple and naked (i.e. without perianth). These combinations of characters distinguish Sanmiguelia from any known group of gymnosperms (Crane, 1988).
Geologic occurrence and age
The Sanmiguelia specimens described here were found near the top of the Trujillo Formation of the Dockum Group of northwest Texas (Cornet, 1986: text-fig. 1). All specimens of Sanmiguelia come from one locality along a dirt road winding down the north wall of Sunday Canyon, just west of Palo Duro Canyon state Park, Texas. The strata containing Sanmiguelia occur just below a sequence of conglomerate and sandstone, and appear to represent a shallowing upwards interdistributary lake deposit on top of a paleosol. The Sanmiguelia colony is restricted to the west end (i.e. shoreline) of a long gray mudstone lens, which is terminated westward by a down-cutting sequence of channel sandstone with conglomerate lag at its base. The lacustrine clam shrimp, Cyzicus sp., can be found in some of the dark gray shale interbeds within the lake sequence.
The remains of Sanmiguelia lewisii were found both in growth position and as fallen axes bearing leaves along bedding planes (Cornet, 1986). The vertical axes were perserved as pith casts surrounded either by carbonaceous residue or petrified wood. Except for small plicate leaves typical of Sanmiguelia on the lower part of the stem (Tidwell et al., 1977), most of the large leaves which characterize the plant had apparently been torn off during burial, leaving only their sheathing leaf bases intact. Attached leaves and leaf fragments in the surrounding siltstone were usually tattered and twisted, reflecting damage during burial. Most of the unisexual inflorescences were found just above the paleosol in which the vertical axes were rooted, and appear to have been knocked down and buried at times of rapid sedimentation (Cornet, 1986). A large apocarpous flower or fruit containing seeds (Nemececkigone fabaforma Cornet) was found caught (preserved) between the nested rachises of Cladophlebis macrophylla fronds, which radiated outwards from a common rhizome, indicating not only that the fern grew alongside Sanmiguelia, but that it was probably situated directly below an inflorescence at the time the fruit dropped.
Palynoflorules from the matrix containing Sanmiguelia and from nearby shales at the locality are identical to those described by Dunay and Fisher (1979) from the upper Dockum Group, and indicate a late Carnian age (Fisher and Dunay, 1984). Dunay and Fisher's (1979) study includes a palynoflorule (7A) from the same locality. Pollen taxa diagnostic for the Late Triassic (Carnian) from the Sanmiguelia locality are listed below. lllustrated taxa are indicated by figure references:
Palynomorphs from the locality diagnostic for the Late Triassic
of Europe:
Cameros porites secatus Leschik 1956
Cyclotriletes margaritatus Mädler 1964 (Fig. 8f)
Enzonalasporites vigens Leschik 1956
Guthoerlisporites cancellosus Playford and Dettmann 1965
Ovalipollis ovalis Krutzsch 1955 (Fig. 7c)
Patinas porites densus Leschik 1956 emend. Scheuring 1970 (Fig. 6i)
Pityosporites devolvens Leschik 1956
Platysaccus triassicus (Maljavkina 1964) Dunay and Fisher 1979
Succinctisporites cf. S.circumdatus Leschik 1955
Vallasporites ignacii Leschik 1956
Palynomorphs from the locality diagnostic for the Late Triassic
of North America:
Alisporites opii Daugherty 1941 emend. Jansonius 1970
Daughertyspora chinleana (Daugherty 1941) Dunay and Fisher 1979
Falcisporites oviformis Dunay and Fisher 1979
Klausipollenites gouldii Dunay and Fisher 1979
Pityosporites oldhamensis Dunay and Fisher 1979
Protodiploxypinus americus Dunay and Fisher 1979
Pyramidosporites traversei Dunay and Fisher 1979
Triadispora dockumensis Dunay and Fisher 1979 (Fig. 6b).
Materials and methods
New specimens of Sanmiguelia (including Axelrodia and Synangispadixis) were discovered on a field trip to Sunday Canyon in September of 1986. The new specimens of reproductive organs are deposited at the Field Museum of Natural History, Chicago, and include:
(1) PP 41768 An incomplete and folded specimen of A.
burgeri inflorescence showing several nearly mature fruits or flowers (not
illustrated).
(2) PP 41769 A large mature terminal flower showing apocarpous
gynoecium, receptacle base, and arrangement of perianth parts (Fig. 1c).
(3) PP 41770 A large mature terminal fruit preserving within its
locules seed casts which - show outlines of
cotyledons; associated with a fern (not illustrated).
(4) PP 41771 A secondary branch of A.burgeri with at least two
closely associated solitary ovuliferous units (one of which is partly pertrified and
yielded a silicified ovule), showing two types of subtending bracts and scale bracts on
the secondary axis (Figs 3e-f, 4f).
(5) PP 41772 An isolated immature carpel compression with preserved
cuticles, paired ovu)es, and transmission tissue (Figs 3b, 4g, 5a-d, 5f-g, 6a, 6d-h,
10c-d).
(6) PP 41773 and PP 41774 Two specimens of S.tidwellii inflorescence
preserving male flowers as compressions (Figs 10a-b, Ilb-c, 12a-c, 13b-c).
Some specimens required degaging in order to reveal hidden parts (Fig. 4). Most specimens, however, provided enough evidence for study and interpretation without any significant preparation. A transfer peel using cellulose acetate film was made of a cluster of well-preserved flower-like units (Figs 3-4). JOEL and lSI-40 SEMs were used (during different periods) to study individual anthers and transfer preparations of large fragments of male flowers (Figs 10-12), and a petrified ovule recovered from one partly silicified carpel (Fig. 3). The samples were sputter coated with less than 250 angstroms of gold. A Tracor Northern energy dispersing spectrometer was used in conjunction with the ISI-40 to detect illite clay inside the ovary of one carpel compression. Portions of male flowers were embedded in plastic, sectioned, and polished. Sections were studied and photographed using a Zeiss reflectance microscope. Standard palynological techniques were used to secure pollen masses, individual pollen grains, and outer cuticle from anthers (Fig. 13), as well as cuticle, transmission tissue, and portions of an ovule from one carpel compression (Figs 5-6). Preparations of pollen and cuticle were studied using a Zeiss binocular microscope and photographed with a Zeiss photomicroscope containing built-in camera. Photographs of the megafossils were made using a Minolta 35 mm camera with enlargement lenses.
Morphological development of Axelrodia carpels was compared with that of Magnolia grandiflora, M. virginiana (Magnoliaceae), Serenoa repens (Arecaceae), and an outgroup conifer, Thuja orientalis. The recent plant specimens were collected during the spring and early summer of 1987 at five-seven day intervals from plants growing in the Houston, Texas area. Megasporophyll length versus two dimensional area and ovule/seed size in mm2 were plotted for Axelrodia, while carpel length versus carpel and ovule/seed weight in milligrams were plotted for the angiosperms. Cone length versus cone and ovule/seed weight in milligrams were plotted for Thuja, because dissection of individual ovule-scale units caused irregular damage and excess fluid loss, and because the scales of one cone differed significantly in size. Weights were obtained on fresh material and on specimens preserved in alcohol using an electronic balance sensitive to 0.001 milligrams at GeoChem Labs. Inc., Houston, TX. Multiple readings were taken until relatively stable (plateau) measurements were obtained due to initial weight loss from evaporating liquids.
Previous interpretation (Cornet, 1986)
The male and female reproductive structures of S. lewisii were borne on separate inflorescences. The female or ovuliferous inflorescence was named Axelrodia burgeri, while the male or polliniferous inflorescence was named Synangispadixis tidwellii. Axelrodia resembled a typical monocot inflorescence (sensu Lilium or Yucca) with its elongate central axis bearing clasping bracts basally that elongated apically to sheathing cataphylls. It undoubtedly terminated a main vegetative axis because of its large size (over 48 cm in length). Secondary branches arose from the axils of cataphylls, possessed numerous spirally-arranged scale bracts, and bore two types of flower-like structures (Figs 1-2): (1) Solitary ovuliferous units consisting of a central megasporophyll (carpel) surrounded by two types of bracts, one peltate and hairy and the other elongate, digitate, and glabrous. Solitary units were each subtended by an elongate conduplicate bract, and they were clustered together in twos or threes along the secondary branches on short tertiary shoots (Fig. la); (2) Large composite flower-like units terminated the secondary branches (Fig. la). Cornet (1986) compared this composite unit to an apocarpous angiosperm flower, because it possessed numerous carpel-like megasporophylls centrally borne on a receptacle, which was subtended by at least two whorls of modified bracts (compare Figs la and 2c).
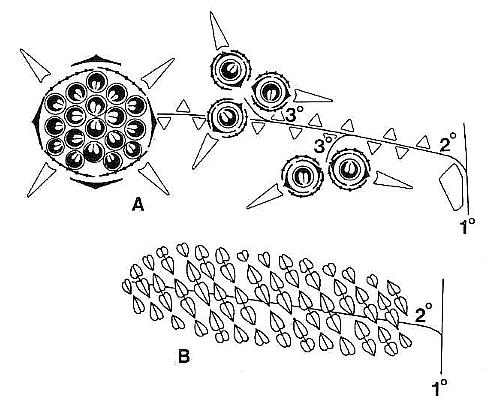
Fig. 1. A and B. Schematic diagrams of Axelrodia burgeri and Synangispadixis tidwellii (after Cornet, 1986). A. Distal part of A. burgeri paniculate inflorescence, showing three branch orders, solitary ovuliferous units on tertiary branches, and composite flower-like ovuliferous unit terminating secondary branch. Three types of bracts are shown in successive 'whorls' with the Outermost 'whorl' on the floral axis being the outermost one: hairy bract = black with keel; digitate bract = black with "beads; conduplicate bract = open elongate triangle; carpel = circle with paired ovules; scale bract = small open triangle; secondary branch shown emerging from axil of cataphyll. B. Portion of S. tidwellii spike or inflorescence, showing two 'branch' orders with secondary 'branch' = floral axis of solitary polliniferous unit. Sessile paired anthers shown as symmetrically opposed and heart-shaped with central sutures; anther pairs shown arranged in spiralling rows.
Synangispadixis resembled an aroid inflorescence (sensu Arisaema) in which naked synangia-like units (Fig. 2a) were spirally borne on an elongate central axis (over 24 cm in length) that probably terminated the main vegetative axis. Synangia-like units were also described as being attached in clusters at the ends of secondary vegetative branches, which bore parallel-veined leaf-like bracts that decreased in size apically down to scale bracts with three veins. Each synangia-like unit bore hundreds of sessile, paired biloculate rnicrosporophylls (Fig. lb). Evidence was presented that indicated enlargement and longation of these units at anthesis.
Axelrodia and Synangispadixis were probably subtended by a large spathe-like vegetative leaf, marking an abrupt transition from vegetative to reproductive axis. Since neither inflorescence was found organically attached to a vegetative axis, even though male synangia-like units were found at the ends of secondary vegetative branches, Cornet (1986) could not determine if Sanmiguelia was monoecious or dioecious. A monoecious habit was implied by the location of fallen male and female inflorescences, which were found lying adjacent to the same cluster of rooted vertical axes.
The megasporophylls were described as carpel-like, with a tapering base, which expanded upwards to a rounded apex and terminated in a bilobed U-shaped stigma-like collar encircling a small canal or opening into a hollow chamber or ovary (Fig. 2b). The megasporophylls were covered with long multicellular and glandular hairs. Two shoulder-like bulges flanked the apex of immature megasporophylls, but became much less prominent on larger megasporophylls.
The microsporophylls were described as anther-like, double-walled with a constricted base, but without filament (sessile). They were borne in symmetrically opposed pairs, and these pairs were arranged in a tight spiral around immature secondary axes (Fig. lb). The paired (biloculate) pollen masses were surrounded by remnants of a tapetum, and the septum separating them largely disappeared at maturity, producing one united pollen chamber containing hundreds of small, elliptical, psilate tectate-granular monosulcate pollen.
Results and revised descriptions
Cornet's (1986) interpretation of Sanmiguelia's reproductive structures was based on a small number of specimens, and therefore suffered from a lack of adequate sampling, but also from the coarse screen size (resolution) of the photographic plates. Those problems are largely corrected with the data described and illustrated below. In addition, some of the previous interpretations were based on a poor understanding of homologous structures. The terms, carpel-like and anther-like in Cornet (1986), are replaced by carpel and anther, respectively, for simplicity of expression, and because their morphology and structure are now well-enough known (see below and Cornet, 1986) to infer homology. As in any fossil material, the assignment of homology is an hypothesis.
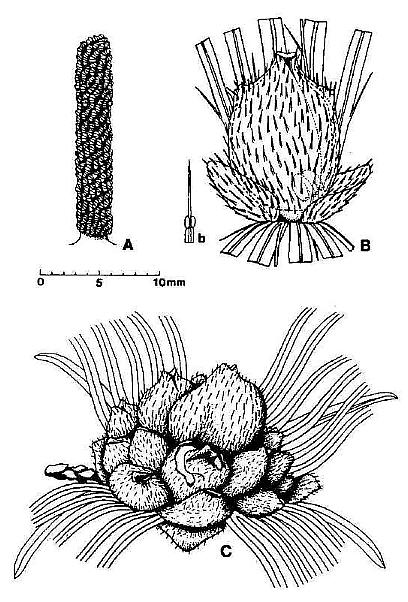
Fig. 2. Reconstructions of Synangispaduis tidwellii solitary polliniferous unit, Axelrodia burgeri solitary ovuliferous unit, and A. burgeri composite flower-like ovuliferous unit.
The study of additional specimens (the subject of this report) and their comparison with associated gnetalean-like reproductive structure (Cornet, 1987a and b) has shown, for example, that the synangia-like secondary branches are in fact solitary flower-like polliniferous units (i.e. male 'flowers'), which are homologues of the solitary flower-like ovuliferous units (compare figs 2a, 2b apd 7a-d): The homology is not at first obvious, because the male and female units are so different in outward appearance, but this homology is all important in understanding how the A.xelrodia carpel evolved and its bearing on angiosperm and parallel gnetalean evolution.
The basic organization and structure of the inflorescences (i.e. Axelrodia and Synangispadixis) have not changed with the study of new specimens. The new specimens support Cornet's (1986) original descriptions, and provide additional documentation for the construction of male and female 'flowers' (the term 'flower' is put in quotes to distinguish solitary ovuliferous and polliniferous units from compound apocarpous flowers borne at the ends of secondary branches). For example, the discovery and study of an isolated megasporophyll compression (Figs 3b, 5a-b) provide support for its carpel-like construction. The discovery of additional solitary ovuliferous units (Fig. 3f) confirms bract morphology as described for the original specimens (Fig.4a-e). Moreover, a partly silicified carpel yielded upon transfer preparation an immature (presumably unfertilized) petrified ovule that shows critical morphological characters (Fig. 3e).
Axelrodia burgeri Cornet
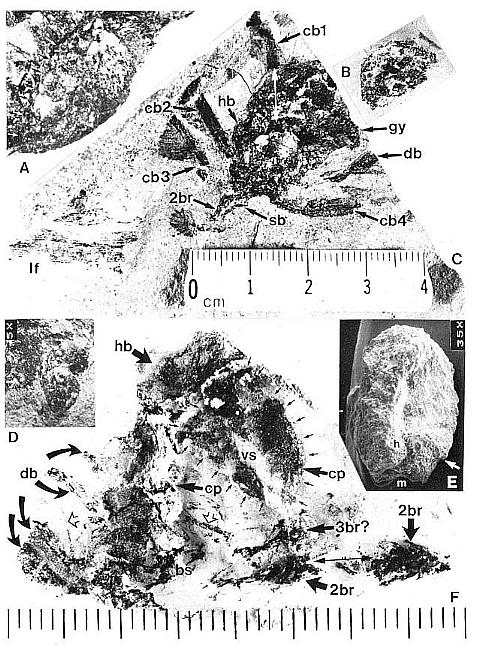
An exceptionally well-displayed specimen of a pre-Cretaceous angiosperm-like flower is illustrated in Figure 3c. It was borne at the end of a secondary branch on an inflorescence, and shows a central gynoecium (gy) comprised of numerous carpels attached to the upper part of a receptacle (an isolated carpel is provided in Fig. 3b for comparison). Portion of the secondary branch (2br) is still attached and bears at least one scale bract (sb). Attached to the lower part of the receptacle are hairy and digitate bracts (hb and db); which are subtended by four conduplicate bracts (cbl-4). One conduplicate bract (cbl) disappears beneath the gynoecium, and its base emerges in a window in the region of the receptacle (white double-headed arrow). Unfortunately this specimen was discovered in a pile of rubble from excavation, and its counterpart was not recovered. Consequently, the number of carpels cannot be determined as in the three-dimensionally preserved gynoecium (Fig. 4a-c). A fragment of Sanmiguelia leaf is also present, which shows at least two orders of parallel venation.
The conduplicate bract is so named because it possessed a strong central keel and V-shaped form that typically caused it to fold in half upon burial. Three of the basal conduplicate bracts in Figure 3c are preserved folded and therefore appear half their normal width. One bract (cb4), however, was rotated upon burial so that it was spread open with its keel prominently displayed as if it were a median vein. Cornet (1986) was able to recognize the morphology of this bract from two specimens (Fig. 4d-e), because sediment was present between the folded halves.
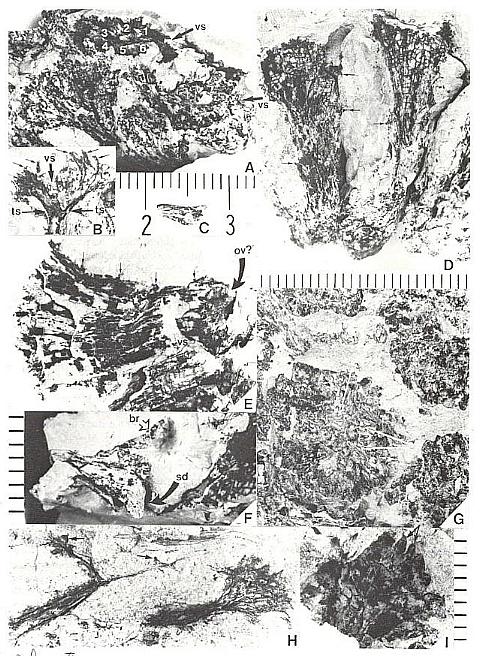
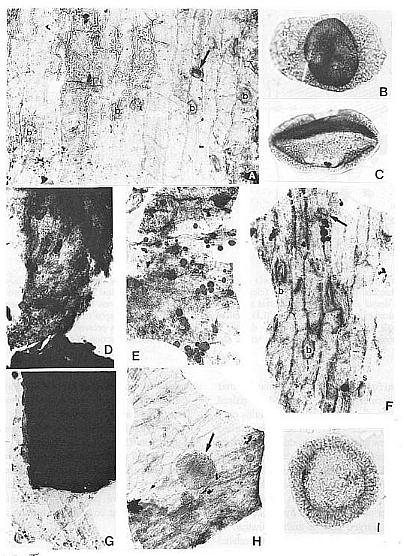
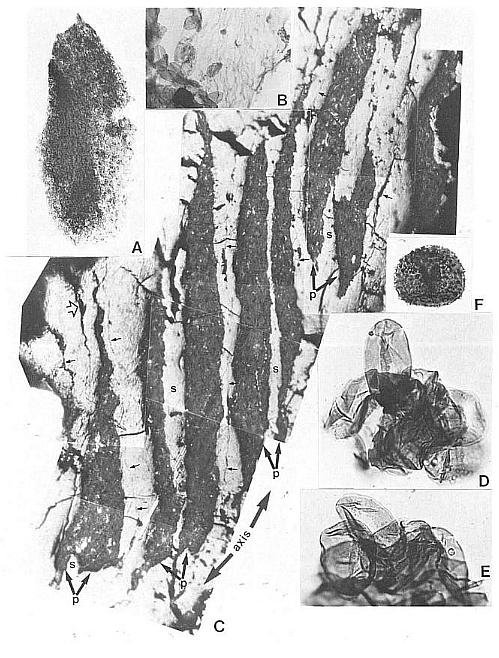
Fig. 3. A-F. Axelrodia burgeri compound and solitary ovuliferous units and their ovules. A. Carpel from three-dimensionally preserved gynoecium in Fig. 4A (scale x 4; arrows denote carpel margin). B. Isolated carpel shown for comparison with overlapping carpels of gynoecium (gy) in adjacent flower (scale x 2). C. Axelrodia burgeri apocarpous flower of the type terminating secondary inflorescence branch, showing four conduplicate bracts (cbl-4) subtending whorl of hairy bracts (hb) and digitate bracts (db); double-headed arrow shows base of cbi emerging in a window through the gynoecium (scale in mm). D. Enlargement of pair of anatropous ovules found at the base of carpel in Fig. 4H (scale x 5). E. SEM of silicified anatropous bitegmic ovule recovered from partly silicified carpel (cp) in Fig. 3F, showing micropyle (m), hilum (h), and distal margin of second integument (arrow; scale x 35). F. Cellulose acetate transfer of two Axelrodia solitary ovuliferous units showing two carpels (cp), secondary branch (2br), one hairy bract (hb), and two digitate glabrous bracts (db), as well as possible tertiary branch (3br?) and spurious appendages (open arrows).
Fig. 4. A-H. Axelrodia burgeri aggregate and solitary ovuliferous units of flowers. A-C. Three-dimensionally preserved apocarpous gynoecium in three different views (see also Cornet, 1986 for drawing of B and indication of perianth). A. Note nearly mature pair of seeds (sd) preserved in a large carpel or fruit, and an immature carpel (arrow; enlarged in Fig. 3A) showing apical stigma (s). B-C. Reverse side of specimen. showing cross section of a carpel with enclosed pair of ovules (arrow). D. Solitary ovuliferous unit or 'flower' (see also Cornet, 1986 for drawing of this specimen) showing immature carpel (arrow), lobes of glabrous digitate bracts (db), and basal conduplicate bract (cb). E. Nearly mature solitary ovuliferous unit or fruit (see also Cornet, 1986 for drawing of this specimen), showing one of two enclosed seeds (sd), apical stigma (s), and basal conduplicate bract (cb). F. Transfer preparation of two solitary ovuliferous units (arrows) adjacent to a secondary branch (2br; enlarged in Fig. 3F); same scale as other flower-like units; labelled are the lobes of two digitate bracts (db) and a hairy bract (hb). G-H. Two isolated Axelrodia burgeri capels (shown at slightly larger scale) represent ovoid (G) and bell-shaped (H) forms (see Cornet, 1986 for drawing of H); labelled are the apical stigmas (s), subapical glands (g), and subbasal paired ovules (0); striate texture of specimen in H due to dense covering of epidermal hairs. All scales in mm.
The morphology of hairy peltate and glabrous digitate bracts was not adequately illustrated and documented by Cornet (1986), although their existence was clear to him from the study of specimens in Figure 4 (a-e). The distinction was obvious due to the presence or absence of cuticular hairs and the digitate versus peltate morphology of the bracts. Figure 3f illustrates a transfer preparation of partly dismembered solitary ovuliferous units or female 'flowers', and again shows the presence of both bract types (db and hb). The peltate hairy bract can be seen more clearly than in other specimens, while the narrow straps with central veins of possibly two digitate bracts merge and unite towards a probable juncture with a carpel (cp on left: partly silicified and incomplete). In addition, there are unusual filament-like structures associated with and attached to the digitate bracts (open arrows), which are shown in Figure 2b.
The morphology of the secondary branches of Axelrodia was not adequately illustrated by Cornet (1986), mainly because only portions of them were visible on the type specimen. The transfer preparation in Figure 3f shows a secondary branch (2br) c1osely associated with two solitary ovuliferous units, and although it is complete between part and counterpart (double-headed arrow), organic connection with the ovuliferous units is not apparent. There is an indication, however, of a possible branch (3br?) connection between the carpel to the right (cp) and the secondary branch. Furthermore, numerous scale bracts are visible on the secondary branch, giving it the appearance of a Pagiophyllum conifer shoot.
The Axelrodia carpel
Cornet (1986) provided evidence that the Axelrodia carpel was originally hollow when he identified a sediment-filled megasporophyll containing possible cross sections of ovules in a three-dimensionally preserved gynoecium (Fig. 4a-c). Since there was only one example that clearly showed these features (other evidence was more circumstantial: e.g. paired seeds within a carpel: Fig. 4a), comparison with angiosperms lacked strength. The isolated carpel compression illustrated in Figure 5a:-b, however, perserves some of the most critical evidence for the homology of its structure with an angiosperm carpel, as well as some unexpected evidence for biotic pollination.
Axelrodia carpels range in form from inverted bell-shaped (Figs 3f and 4h) to egg-shaped (Figs 3a and 4g). A stigma-like apex is present on all carpels, but is sometimes spread apart on the bell-shaped form probably due to distortion during compression (Fig. 4h). Two gland-like swellings create shoulders or pronounced appendages distally (g), and appear to be symmetrically (bilaterally?) positioned on opposite sides where the carpel begins to taper towards the stigma-like apex. These swellings are more pronounced on the inverted bell-shaped form (Fig. 4h), leading Cornet (1986) to speculate that their development anticipated the enlargement of the ovary to accommodate developing seeds. The new specimens (Figs 3f and 4g) indicate, however, that differences in form may be related to the degree of gland development, because the more carpel-like form (Figs 4g and 5a-b) appears to owe its shape to three small glands (evidenced by ovoid organic stains in the matrix under the glands) distributed across the dorsal side instead of two large glands (Fig. 4h).
Evidence for a carpel wall
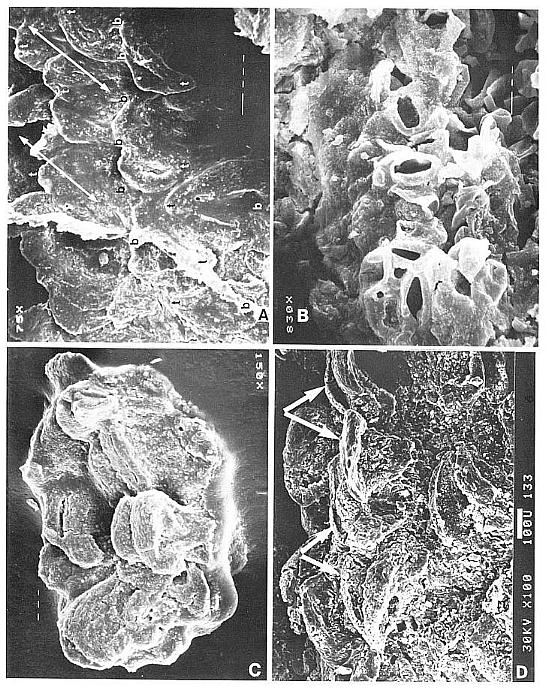
Oxidation and clearing of pieces of a carpel compression in a basic solution (Fig. 5a-b) yielded several different types of tissue or cuticle. The most obvious cuticle is from the outer wall, which is thicker than the inner cuticle, possesses well-defined rectilinear cell outlines and a fine tread-like pattern (Figs 5c, 6a, 6f and 6h), and reveals the circular to elliptical bases of hairs (b), which are usually situated within the boundaries of epidermal cells. When hairs are recovered in cuticle preparations, they are usually long, thick-walled, and sometimes aggregated (Fig. 5e) rather than widely spaced as the distribution of hair bases would suggest. There are also narrow lens-shaped structures with a prominent median thickening, which may represent either stomata or glands (s). Pollen can be found adhering to the outer cuticle; the most common type is monosulcate like that of Synangispadixis (Fig. 6a and 6f., arrows), but other types are also present, such as Patinasporites densus (compare Fig. 6h and 6i). The inner wall cuticle, however, is much thinner, tends to fold rather than break, and possesses in some areas numerous closely-spaced stomata (Fig. 5d). The greater abundance of stomata inside the ovary wall may be related to humidity control in a semi-arid climate or to fluid volume control within the ovary. A secretion or mucilage, typically fills the ovary in the Chloranthaceae (Endress, 1987a) and Araceae (personal observation). Stomata on both inner and outer carpel-wall cuticles support a leaf origin.
Evidence for a pollen tube transmission tissue
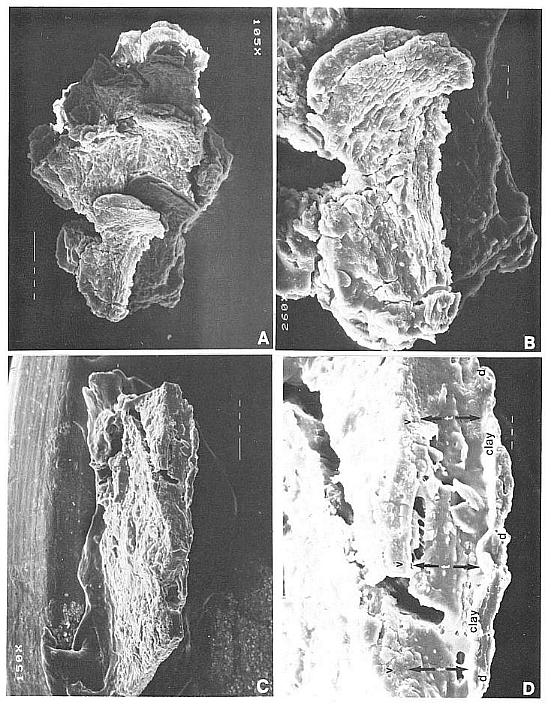
A third type of tissue or organ is preserved between the cuticles of the dorsal and ventral walls. It can be demonstrated in pieces of compression viewed in cross section under SEM (Fig. 10c-d). A middle layer (t in Fig. 10d) can be seen to originate and thicken from left to right in Figure 10c. This layer is attached or appressed to the ventral wall (v) and separated by a thin layer of illite clay (SEM elemental spot analysis) from the dorsal wall (d). The clay occupies what volume remains of the collapsed ovary cavity, and probably entered the ovary at the time of burial (cf. Cornet, 1986). The position of the middle layer on the ventral side of the carpel was determined by observing the position of the white clay layer (obvious against the coal-black compression) in place on the specimen (Fig. 5a-b), and its relationship to the side of the compression containing the impression of the ventral suture. The distribution of the middle layer (t) was followed through the compression (possible because of the distribution of compression fragments: Fig. 5a-b) and plotted. It is shown in Figure 9a as the area with the diagonal pattern. It clearly straddles the ventral suture and extends from near the stigma-like carpel apex basally to include the area occupied by two ovules (Fig. 5a, o), one on either side of the ventral suture (Fig. 9a). The ovules were identified as an additional layer by their size and ovoid shape within the compression, and by oxidizing and clearing a piece of one of them (Fig. 6d and 6g).
The middle layer (t), when isolated, oxidized and cleared, has a very distinctive fibrous structure or texture (Figs 5g and 6d). Beads of resin-like material were found situated between the middle layer and the ventral wall (Fig. 6e), and also adhering to or within the middle layer (Fig. 6d). The distribution of this layer down to the ovules is documented in Figure 6d and 6g, which show this layer in contact with the thick compression of multiple cuticles belonging to one ovule. The exposed surface of the middle layer facing the ovary cavity was visible in one compression fragment under SEM (Fig. 5t). Its surface is covered by thousands of minute papillae arranged in rows. The rows give the middle layer its fiber-like texture. They are oriented vertically or baso-apically within the carpel; their orienation was determined by the orientation of the compression fragment in Figure 5f, which is located by the x in Figure 5a.
The middle layer appears to be divided by the ventral groove into two bilaterally-symmetrical elongate scale-like organs which were appressed to the ventral wall and extended from the area of ovule placentation apically to the base of the stigma (Fig.9a-b). The papillate character of their outer surface, the orientation of those papillae in vertical rows aligning stigma with ovules, and the distinction of this layer from any ovular integument (or micropyle) by direct comparison (Fig. 6d and 6g) strongly suggest that these paired organs functioned as a pollen tube transmission tissue. The presence of resin-like material within the middle layer and ventral wall (Fig. 6d-e) suggests the production of a non-polar terpenoid resin that probably had toxic properties when fresh and fluid, and may have been a defense against attack by pathogens, herbivores or seed predators (Armbruster, 1984). There is no indication of pollen either within the ovary or clinging to the side of the middle layer facing the ovary. No pollen was found trapped between the cuticles of the middle layer, which would probably have occurred if the transmission tissue contained fluid-filled passages or channels connecting mouth and micropyles as in Caytonia and Glossopteris (Reymanowna, 1973; Gould and Delevoryas, 1977; Crane, 1985). A polar fluid containing polysaccharides and carbohydrates for pollen tube growth is unlikely to survive fossilization, while resin-like material within the middle layer probably would survive fossilization. The similarity of the massive middle layer to the more massive types of transmission tissue possessed by the Chloranthaceae (e.g. Hedyosmum mexicanum and Ascarina lucida: Endress, 1987a) and some Araceae (e.g. Anthurium, personal observation) is consistent with the low phylogenetic position suggested for the Chloranthaceae and Arales (Endress et al., 1987a; Dahlgren et al., 1985).
The presence of transmission tissue on the outer surface of Axelrodia stigmas has not been verified, although a modified cuticle does exist there (Cornet, 1986: p. 260). The mapped termination of the middle layer just before reaching the stigma (Fig. 9a) may be an error due to the inability to follow this layer below a certain thickness. Transmission tissue exudate (cf. Cresti et al., 1986; Endress, 1987a) may have filled the short stylar canal and wetted the stigmatic surface, thereby bridging any gap between pollen landing surface and transmission tissue in Axelrodia. Conversely, an abscence of the middle layer from the stigma may be a primitive characteristic, which correlates with a psilate pollen grain lacking a porous exine structure indicative of a sporophytic incompatibility mechanism (Zavada, 1984; Zavada and Taylor, 1986). Sporophytic self-incompatibility requires (1) physical contact between a porous pollen exine and transmission tissue so that sporophytic substances produced by the anther tapetum and carried by the exine can influence the gametophytic system directly (Zavada, 1984), and (2) chemical agents produced by the tapetum that will influence compatibility. Consequently, a sporophytic incompatibility mechanism and a porous exine (i.e. reticulate-columellate) probably evolved after transmission tissue formed its own stigma or stigmatic surface above the carpel as in Hedyosmum, Ascarina, Chloranthus, etc. (Endress, 1987a).
Evidence for bitegmic ovules
Pieces of an ovule recovered from the carpel compression in Figure 5a-b are comprised of numerous layers of thin cuticle, so many in fact that oxidation and clearing could not make them transparent (Fig. 6g). Although the exact number could not be determined, the layered edges of broken ovular compression suggest as many as ten cuticles in cross section, more than can be accounted for by a unitegmic ovule with enclosed nucellus.
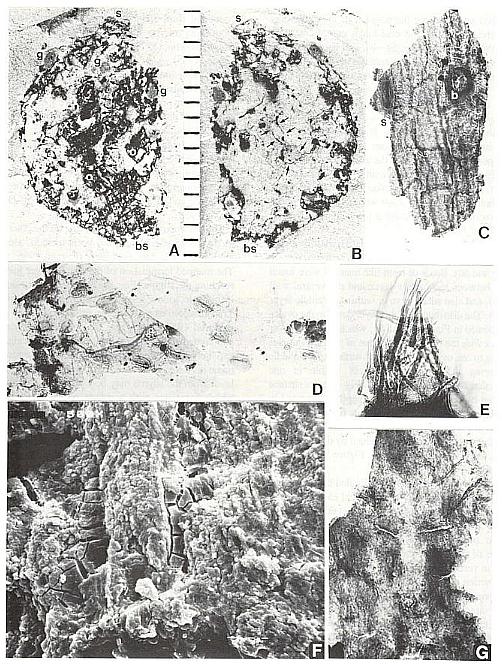
Fig. 5. A-G. Axelrodia burgeri carpel and its various organs and cuticles (figures approximately x 500 unless otherwise indicated). A-B. Isolated carpel showing part and counterpart (scale in mm); small arrows (Fig. 8B) point to arcuate cuts in dorsal wall cuticle; bs = carpel base, s = apical stigma, g = subapical protuberances interpreted as glands because they overlie organically stained matrix, o = positions of ovules (see also Fig. 5A-B). C. Thick outer cuticle showing tread-mark pattern, cell outlines, possible stomata or gland (s), and base to an epidermal hair (b). D. Thin, folded dorsal-wall inner cuticle (facing ovary) showing faint cell outlines and abundant stomata. E. Cluster of epidermal hairs like the type largely removed from the carpel in A-B., F. SEM view of surface (facing ovary cavity) of middle layer of transmission tissue, showing thousands of minute papillae aligned in vertical rows. G. Transmitted light view of middle layer, showing the same pattern of minute papillae.
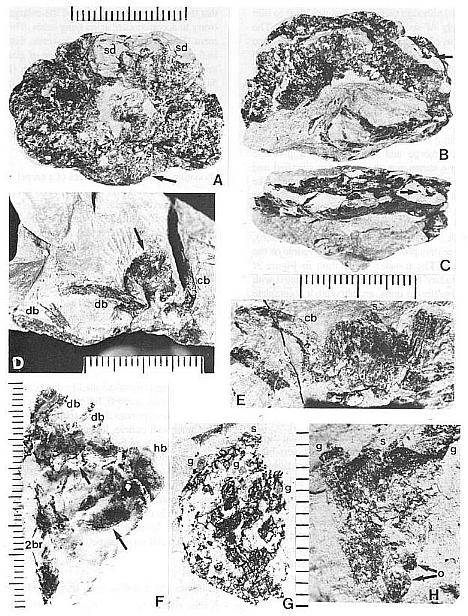
Fig. 6. A-I. New gnitalean like 'flowers' belonging to urusexual inflorescences or spikes from the Sanmiguelia locality. A. Distal end of female spike showing apical compression of two 'flowers' (arrows pointing to ventral sutures: vs), and lateral compression of several other 'flowers'; a ring of interseminal scales (numbered) is visible in the apical compressions; scale in mm. B. Bell-shaped apex of a female 'flower' showing ventral suture (vs at arrow) spread open, gland-like structures (small arrows) around rim of cupule, and tops of enclosed interseminal scales (ts at arrows) forming a carbonaceous 'bridge' across cupule; scale X4. C. Isolated seed cast; scale in mm. D. Two trumpet-shaped female 'flowers' attached to spike axis; apices to 'flowers' incomplete; small gland-like-structures on tubular part of cupule visible at arrows; scale in mm. E. Lateral compression of female 'flower' showing interseminal scales separated by matrix on one side of cupule, sediment fIlling space once occupied by a seed, small gland-like structures on tubular part of cupule (small arrow), position of ventral suture (vs), and a possible non-functional ovule (ov?) at base of cupule replaced by siderite; scale X6.2. F. Base of a female 'flower' showing sediment cast of seed (sd) and a small lobate bract attached to base of cupuIe; scale in mm. G. Portions of four male 'flowers' compressed baso-apically and showing crowded bivalved microsporophylls attached to outside of cupule; fiber-like structures or hairs visible within one cupule (arrows); scale X3. H. Cross sections of two male 'flowers' showing tubular base and bell-shaped apex; most of the bivalved microsporophylls pulled off during burial and are 'floating' in matrix between 'flowers'; note tattered margins of cupule and presence of some microsporophyll bases on inside of rim to cupule (arrows); scale X4. I. Bell-shaped apex to a male 'flower' showing bivalved microsporophylls attached to rim of cupule (a pair of bracts to one microsporophyll at arrows); scale in mm.
The most convincing evidence for two integuments comes from a silicified ovule (Fig. 3e), recovered during the transfer of solitary 'flowers' to cellulose acetate film (Fig. 3t). The ovule is about 1.0 mm long. A distinctive recurved funicle or raphe (i.e. ridge) can be identified, substantiating an anatropous condition, and a large micropyle (Fig. 3e, m) can be seen at one end adjacent to the hilum (Fig. 3e, h). An inner integument forms the border of the micropyle, while the distal end of the outer integument can be recognized by its raised margin (Fig. 3e, arrow). Normally the outer integument of angiosperms extends beyond the inner one. Ovules with a prominent inner integument, which projects well beyond the outer, are occasional, as in the Chloranthaceae (e.g. Chloranthus, Hedyosmum), Annonaceae, Trapaceae, Proteaceae, and some of the Cactaceae (Ean1es, 1961; Endress, 1987a). This specimen compares in size and morphology with an unfertilized, anatropous, bitegmic angiosperm ovule.
Evidence for biotic pollination
The carpel compression illustrated in Figure 5a-b is preserved as both part and counterpart, but it is badly cracked and divided between the two halves. The manner of preservation allows both sides to be observed as impressions in the matrix. Figure 9a gives locations of pieces of cuticle and ovular integument removed for scanning electron microscopy (SEM) or for transmitted light microscopy (SLD). The matrix impression contains organs and structures that were attached or adhering to the outer cuticle. The position of the ventral suture can be followed from the cleft stigma basally as a groove or crease to the position of the ovules (compare Figs 5a, arrows, and 9a). Its arcuate shape is probably due to the collapse of the ovary. A similar groove or indentation marking the position of the ventral suture can be seen in a carpel cross section (Fig. 4b, above arrow). In addition to a few epidermal hairs embedded in the matrix (most hairs are missing or preserved only as hair bases on the outer cuticle: Figs 5c, 6a and 6t), there are fragments of cuticle from the edges of tears in the carpel wall (FIg. 5b-c, arrows), and fragments of Synangispadixis anthers (Fig. 9a, black and white tear-drop shaped objects; the black fragments are on the ventral side, the white ones on the dorsal side).
Most of the anther fragments are the distinctive thick-walled borders to the distal suture (e.g. Fig. 10a-b), and may have been transported to the carpel as the indigestible portions of an insect's meal. The tears in the carpel wall have a distinctive arcuate shape suggestive of cuts that could have been made by a chewing insect's mandibles. Most of the cuts are on the dorsal side of the carpel, which had a thinner wall (Fig: 8b), while most of the anther fragments are aligned in a row on the ventral side as if deposited along an insect's path. The presence of resin-like material between the cuticles of the carpel wall, and the presence of large specialized glands situated near the stigma suggest the presence of both a pollinator reward system (i.e. nectar or nutrient) and a defense system (i.e. antibacterial, fungicidal, and antiherbivor toxins: Armbruster, 1984). Finally, the carpel was physically or physiologically severed from its 'flower', and the jagged edge also could have been caused by the chewing action of an insect. If phytophagous insects were the primary pollinating vector, perhaps the formation of a terminal apocarpous flower is an evolutionary response to counteract the attacks on solitary ovuliferous units.
Floral phyllotaxis of the compound apocarpous flower
The organization of floral organs is similar between solitary ovuliferous units borne on tertiary branches and the large apocarpous flower borne at the ends of secondary branches (Fig. la), with each organ type maintaining the same relative position in the floral structure even though tire number of parts increased disproportionately in the apocarpous flower. Whereas the solitary 'flowers' had one carpel, one conduplicate bract, and probably two hairy and two glabrous bracts, the apocarpous flower had from few to many carpels subtended and surrounded by an indeterminate number of hairy and glabrous bracts, which were themselves subtended by a pseudowhorl or whorl of four elongate conduplicate bracts. An ordered arrangement of carpels is not apparent from the study of a three-dimensionally preserved gynoecium (Fig. 4a-c), and the reconstruction in Figure 2c suggests that not all carpels were oriented anteriorly, possibly indicating an origin from more than one floral primordium. Conversely, variable orientation of carpels may be a primitive character, since it also occurs in the Chloranthaceae (Endress, 1987a).
Angiosperm flowers with a small number of perianth parts typically have gynoecia with few carpels which are usually whorled or fused (Endress, 1987b). Unisexual female flowers of some Alismataceae and Ranunculaceae are an exception. In these flowers a large apocarpous gynoecium probably evolved from an increase in number of carpel initials (Dahlgren et al., 1986). Axelrodia apocarpous flowers show a decrease in number of parts basally for each organ type, allowing for a more ordered arrangement basally (cf. Endress, 1987b). The origin of a poorly organized gynoecium with a variable number of carpels may be related as much to extrinsic factors (e.g. the type and effectiveness of biological pollinators) as to intrinsic ones (e.g. an origin from the condensation of numerous floral primordia). The presence of both solitary and compound floral types in Sanmiguelia may be the first clear evidence that the polycarpous angiosperm flower (and its derivatives) is not directly homologous with gnetalean and bennettitalean 'flowers'. The simple flowers of the Chloranthaceae (Endress, 1987a) and Piperales may be the exception (Burger, 1977).
Origin of a closed carpel
At the Sunday Canyon locality isolated leaves of Pelourdea poleoensis were found in the paleosol underlying the Sanmiguelia root zone and in thin shales interbedded within the sandstone sequence overlying the Sanmiguelia colony. The leaves of both taxa are frequently reported from the same localities (Ash, 1987a). Both plants had an herbaceous habit, grew to a similar overall size, possessed large leaves with parallel venation and clasping leaf bases, and lived in the same habitat (Ash, 1987a, 1987b), suggesting a close ancestral relationship. Five unisexual reproductive axes or inflorescences bearing gnetalean-like 'flowers' were found in the paleosol just below the Sanmiguelia colony (cf. Cornet, 1986), while an additional one was found within the clayey siltstone beds entombing the Sanmiguelia colony (Fig. 8a-i: Cornet, 1987a; In Prep.) They are all spikes possessing a thick central axis with a large pith cast, like that of Pelourdea and Sanmiguelia (Ash, 1987b; Cornet, 1986). Four long male spikes were found in a block of siltstone next to a large axis and long lanceolate leaf of P. poleoensis. They are oriented in the same direction as if attached to a common axis, but the adjacent block which would show an organic connection was mistakenly not collected. The scarcity of Sanmiguelia leaves in the paleosol, organic connection between Synangispadixis and Sanmiguelia, and the association of Axelrodia with a large leaf of Sanmiguelia (Cornet, 1986) indicate that the new reproductive structures do not belong to Sanmiguelia. They probably belong to Pelourdea, based in part on the large number of vegetative parallels between Pelourdea and Sanmiguelia, in part on additional parallels between Axelrodia, Synangispadixis, and the new reproductive structures (described below), and in part by the process of elimination, since no other 'autochthonous' seed plants have been identified in the Sanmiguelia facies zone.
The gnetalean-like 'flowers' of the new inflorescences are remarkably similar in form to the inverted bell-shaped variation of the Axelrodia carpel (compare Figs 7b and 7d; 4h and 8d, e), warranting a more detailed description and comparison: male or female 'bisporangiate' inflorescences, at least 13 cm in length and differing mainly in the functional development of either ovules or pollen sacs, bear up to one hundred, 1.5 cm long, spirally-arranged tubular trumpet-shaped 'flowers' with multitier lobate margins (Figs 7c-d; 8a-i). Each trumpet-shaped 'flower' has a ventral or anterior suture which is frequently 'open' or spread apart at the bell-shaped apex (Fig. 8a and b, vs at arrows), but usually closed along the floral tube (Figs 7c-d, 8e, vs). Attached to the outside of the male 'flower' (Fig. 7c) are hundreds of bivalved microsporophylls (Figs 8g-i), each with a central elongate pollen sac or hollow stalk that divides apically into a cluster of 4-6 small sacs, which contain small (18-25 um), oval, psilate monosulcate pollen. Microsporophyll construction is very different from that of Synangispadixis, but very similar to that of Ephedra. In Synangispadixis the paired bract homologues are each apparently folded to enclose a pair of pollen sacs (creating the tetraloculate condition in angiosperms), while in the new plant the bracts remained free. Inside the narrow tubular base of the gnetalean-like male 'flower' is a cluster of thick fibers or hairs (Fig. 8g, arrows).
Inside the gnetalean-like female 'flower' (Figs 7d; 8d) a single central ovule with a long narrow apex (Figs. 8c; 8f, sd) was surrounded by a ring of about six sterile scales (Fig. 8a, e). These scales, which are reminiscent of the interseminal scales of the Bennettitales, rise to the top of the tubular part of the cupule (Fig. 8b, ts),where they form slightly expanded heads at the base of the inverted-bell-shaped floral apex (Fig. 8a, numbered in one of two apical views indicated by arrows). The apex of the seed (micropyle?) appears to extend to near the top of the surrounding scales. Possible aborted or non-functional ovules occur next to the central ovule or sediment-filled cavity at the base of some cupules (Fig. 8e, ov?). Along the lower part of the female 'flower' possibly four or five lanceolate to semi-digitate scale bracts insert around th4 floral tube (Fig. 8f, br) below small gland-like structures (Figs. 8d and e, small arrows). These gland-like structures may represent sterile microsporophyll homologues, based on an attachment identical to the microsporophylls of male 'flowers'. They increase in number and size around the cup-shaped apex of the female 'flower', forming a dense cluster of ovoid bodies supported by narrow filaments (Figs 7d; 8b, small arrows).
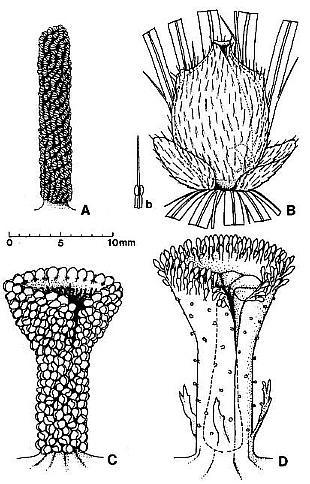
Fig. 7. A-D. Reconstructions of angiosperm and gnetalean 'flowers' from the Sanmiguelia locality. A. Synangispadixis tidwellii male 'flower' showing a lack of perianth, and crowded paired microsporophylls attached to the outside of an apically constricted or closed, tubular (i.e. cupulate) floral axis. B. Axelrodia burgeri female 'flower' showing two types of perianth bracts, an associated glandular organ (Fig. 7b) which was probably attached to a digitate bract, and a cupulate structure (i.e. carpel). with subapical glands, a constricted apex with ventral suture forming a stigma, and a pair of subbasal anatropous bitegmic ovules enclosed within an ovary. C. Gnetalean male 'flower' showing a lack of perianth, a ventral suture, and crowded bivalved microsporophylls attached to the outside of a cupulate structure. D. Gnetalean female 'flower' showing a ventral suture, glands instead of microsporophylls attached to the outside of a cupulate structure, ring of sterile scales surrounding cavity where a single seed developed, and small digitate bracts forming a rudimentary perianth.
The parallel morphology between Synangispadixis and Axelrodia 'flowers', on the one hand, and between the male and female gnetalean-like 'flowers' on the other becomes apparent when they are compared to each other (Fig. 7a-d). Both types of male 'flowers' are naked (lacking basal bract-like appendages) and covered with microsporophylls, while both types of female 'flowers' possess basal bract-like appendages, gland-like organs (instead of microsporophylls) which are concentrated apically, ventral sutures, and one versus two functional qvules attached near or at the base of a cupulate structure. The similarity in form between some Axelrodia carpels (e.g, Figs 3f and 4h) and the basic copstruction of the gnetalean-like male and female 'flowers' suggests a fundamental and ancestral relationship as predicted by cladistic analyses for the GnetaIes and angiosperms (Crane, 1985; Doyle and Donoghue, 1986, 1987a).
The origin of the closed carpel in Axelrodia can be conceived as a parallel development between male and female 'flowers'. A false or closed tubular axis and a constricted apex (i.e. not flared) for the Synangispadixis 'flower' (Fig. 7a) are implied by comparison with the parallel morphology of tubular trumpet-shaped gnetalean-like 'flowers', and to a lesser extent by its enlargement in breadth during anthesis and the lack of a vascular core (Cornet, 1986; Figs 11a-c and 13c). Similarly, the female Axelrodia 'flower' (Fig. 7b) has a constricted apex that forms an ovary and creates the necessity for pollen to land and germinate on carpellary tissue. Although the 'flowers' of Axelrodia and Synangispadixis outwardly do not appear homologous, comparison with the associated gnetalean-like 'flowers' suggest that they are fundamentally similar and bisexual as in extant Gnetales and the extinct Bennettitales (Crane, 1985; Doyle and Donoghue, 1987a, 1987b).
Synangispadixis tidwellii Cornet
Two additional specimens of S. tidwellii were discovered in 1986, and each is preserved as a compression containing murnniified anthers (Fig. 11b-c). Cornet (1986) described the male 'flowers' as secondary branches terminated by synangia-like organs. The male 'flowers' are now recognized as probable homologues of Axelrodia solitary ovuliferous units or female 'flowers' (compare either Figs 2a-b or 7 a-b). The specimen illustrated in Figure 11c has an inflorescence axis that bifurcates or branches near its end (not shown), a characteristic which was not observed by Cornet (1986) and which reduces the morphological gap between the female inflorescence or panicle and the male inflorescence or spike (see reconstructions in Cornet, 1986; Fig. 6). In all specimens of S. tidwellii the 'flowers' insert spirally around the main axis, and there are no bracts or perianth-like structures evident. The holotype (Fig. 11a) shows acropetal maturation with mature male 'flowers' restricted to the lower part of the inflorescence axis. Apical anthers of undehisced 'flowers' are less mature than basal anthers (Cornet, 1986). Dehisced 'flowers' at the base of the inflorescence (Fig. 11a) have a longer than usual, naked lower floral axis that may be the result of deciduous loss of anthers after dehiscence.
The male 'flower' and its homology with angiosperm stamen
The homology of male and female solitary 'flowers' and their similarity to associated gnetalean-like 'flowers' (Fig. 7c-d) suggest that the male floral axis is in fact a closed tube or false axis. The axis, when visible, is preserved as a thin carbonaceous layer with no apparent vascular core (Fig. 11 c), which would be expected if it was ontogenetically 'hollow'. The possible origin of both the Axelrodia carpel and Synangispadixis floral axis from homologous tubular or cupulate structures gives new vitality and meaning to the classical or traditional comparison of the laminar stamen and carpel to unfolded and folded leaves, respectively (Canright, 1952; Bailey and Swamy, 1954; Eames, 1961). The major difference between the Synangispadixis 'flower' and the laminar stamen is that the former posseses hundreds of paired sessile biloculate anthers, while the latter contains only one pair. That difference, however, can be easily rectified through reduction. Few angiosperms produce stamen with more than two anthers, and those that do usually have no more than eight (Eames, 1961). The reduction to one pair may be related to the timing of anthesis, which could not be synchronized or limited to a short period of time with acrofloral anther maturation as in Synangispadixis. Investment in one enlarged pair of anthers instead of hundreds of small ones may be a specialization related to more selective insect pollinators, or to interspecific competition for those pollinators.
Fig. 8. A and D-H. Fragments of acid-resistant cuticles of Axelrodia burgeri carpel (all figures approximately x 500). A. Thicker outer carpel-wall cuticle showing circular bases to hairs (b) and a monosulcate pollen grain (arrow). D. Ventral transmission tissue (middle layer) in contact with ovule. E. Resin-like bodies clinging to inside of outer ovary-wall cuticle, which shows typical tread-like pattern. F. Thicker outer carpel-wall cuticle showing circular bases to hairs (b), possible glands or stomata (s), and a monosulcate pollen grain (arrow). G. Ovule showing integuments along broken edges (black due to sandwich of at least eight layers), and a fragment of adhering dorsal ovary-wall cuticle. H. Appressed inner and outer ventral-wall cuticles (note crossing cell pattern), pollen tube transmission cuticle from middle layer (darker with resin-like beads), and a Patinasporites densus grain (31 um diam.) adhering to the outer carpel-wall cuticle (arrows). Fig. 6B. Triadispora dockumensis, 49 um wide. Fig. 6C. Ovalpollis ovalis, 48 um wide. Fig. 61. Patinasporites densus, 40 um in diameter.
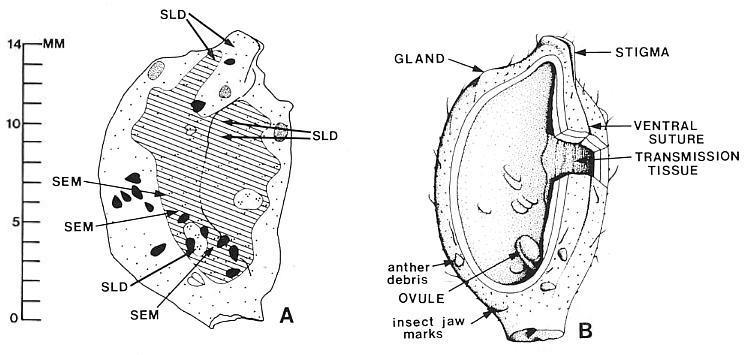
Fig. 9. A and B. Isolated Axelrodia burgeri carpels (Fig. 5A-B). A. Camera lucida drawing of carpel showing distribution of middle layer (cross-hatched pattern). two ovules (clear ovoid areas within cross-hatched pattern), ventral groove or suture, " anther fragments on dorsal side (white) and ventral side (black) of carpel, and positions from which fragments of carpel compression were taken to make cuticle slides (SLD) and sc1anmng electron, micrographs (SEM). Dot pattern: one dot = only dorsal and ventral wall layers present; two dots = dorsal, ventral; and middle layers present; three dots = dorsal, ventral, middle, and ovule layers present; dots in position where data recorded. B. Reconstruction of carpel in A with interpretations (see text).
Some more advanced angiosperms produce stamen bundles, which are best known in the Guttiferae and in certain Australian genera of Myrtaceae (Stebbins, 1974). Stamen bundles may be the only functional analogues to Synangispadixis 'flowers' among extant angiosperms, giving an insect the illusion of a greater food or pollen source without sacrificing anthesis timing. Based on the homology of carpel and stamen, syncarpous gynoecia and stamen bundles in angiosperms could be homoplastic structures.
Primitive laminar stamen are recognized with either abaxial, adaxial, or marginal anther attachment (Canright, 1952; Eames, 1961). Evolution from a tube or cylinder allows for virtually any type of attachment. Chloranthoid androecia with cylindrical stamen and variable anther attachment are known from the Early Cretaceous (Friis et at., 1986). Furthermore, the presence of a ventral suture on the male gnetalean-like 'flower' (Fig. 7c) implies that the Synangispadixis 'flower' also possessed a closed ventral suture, giving the floral axis the genetic option of either unfolding or flattening to produce a laminar structure, or remaining cylinder to produce the narrow filament so common among angiosperms.
The Synangispadixis anther
Synangispadixis anthers are arranged in spiral rows around the flora axis, and occur in opposite pairs as in angiosperms (Fig. 1b; Cornet, 1986). The new material clearly shows this arrangement (Figs 10a, 12a, 12c). The anthers in Figure 10a conveniently possess contrasting electron densities in different rows. The tip to tip (t) and base to base (b) arrangement is apparent in Figure 122a and 12d, while it is not as apparent for narrow-elongate or immature anthers with poor tip and base (abaxial/adaxial?) differentiation (Figs 10a, 12c). The paired arrangement is not only critical to an angiospermous affinity, but also to an ancestral relationship with sister groups, the Gnetales and Bennettitales (Doyle and Donoghue, 1987a), for which the basic microsporophyll construction is similar in having a distinct bivalved (paired) construction.
Fig. 10. A and B. Synangispadixis tidwellii microsporangia or anthers; note alternating electron density for anthers belonging to different rows on flora axis in A; enlargement of one anther in B. Fig. 10C and D. SEM view of piece of Axelrodia burgeri carpel compression (from specimen in Fig. 8A-B), with ventral surface up (to the left); note decrease in thickness from right to left (from up to down) due to disappearance of middle layer in C, and separation of dorsal wall (d) from adnate ventral wall (v) and middle layer (t) by illite clay layer in D. Mounting glue forms threads bridging fractures. SEM magnification on figures.
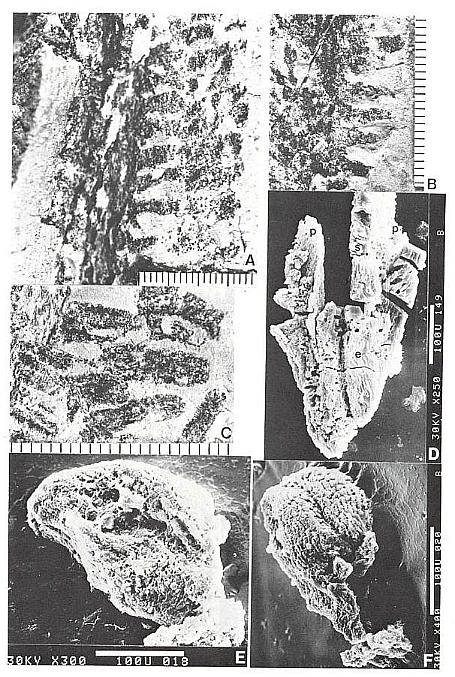
Fig. 11. A-F. Three specimens of Synangispadixis tidwellii spikes or inflorescence and two isolated anthers. A. Holotype showing mature dehisced male 'flowers' along lower part of inflorescence axis (see Cornet, 1986 for more complete illustration and reconstruction). B. Portion of immature inflorescence axis bearing 'flowers' and anthers illustrated in Figs 10A-B, 12C and 13C. C. Portion of mature inflorescence axis bearing anthers and pollen illustrated in Figs 12A, 12B and 13B. D-F. SEM views of two anthers (D and E-F) isolated from holotype, showing sessile base, distinctive adaxial or ventral suture flanked by enlarged endothecial cell (see Cornet, 1986 for drawings and additional documentation), two pollen masses (p) separated by a septum (s), and a wrinkled epidermis (e) (Fig. 13B) that is typically present or preserved below the thick-walled suture margins. Scales in mm for A-C; 100 micron scale bars for D-F.
Fig. 12. A and C-D. Close-ups of Synangispadixis tidwellii anthers, showing arrangement in rows (A and C) and paired arrangement with apical tips (t) and bases (b) opposed (A and D, double arrows). B. SEM view of uncompressed pollen within a mature anther; tapetal debris concealing some pollen grains. Magnification and scale bars indicated on figures.
Fig. 13. A. Pollen mass from an anther of Synangispadixis tidwellii, showing no pollen-sac wall (about x 200). B. Epidermal cuticle isolated from an anther, showing abundant monosulcate pollen clinging to one side (about x 500). C. Trans-longitudinal section through portion of a male 'flower' and viewed with reflected light, showing dark pollen masses organized in pairs (P) within each anther and separated by septa (s); the walls of adjacent anthers separated by thin cracks or lines (small arrows); a ventral suture to one anther is visible at lower left (open arrow); see text for further description. D-E. Pollen from S. tidwellii anthers, showing a monosulcate aperture and a faint intragranular exine structure. F. Cyclotriletes margaritatus (Carnian), which is identical to spores recovered in situ from osmundaceous fertile spikes associated with Cladophlebis macrophylla fronds and rhizomes at the Sanmiguelia locality.
Synangispadixis microsporophylls are variable in shape, ranging from lunate to tear-drop to narrow-elongate (Figs 10a-b; 11d-f; 12a, 12c-d). They range in length (i.e. tip to base) from 230 to 525 microns, and in height from 170 to 407 microns. Their size is undoubtedly reduced from that in life due to compression and shrinkage during coalification. Most are longer (tip to base) than tall (Fig. 11e-t), but some are taller than long (Fig.10b). Regardless of shape they all have a pronounced thickening of cells (referred to here as the anther cap) bordering a median suture, which extends from the apex across to the opposite side of the anther, where it seems to disappear as the anther cap terminates. Some specimens indicate that the suture continues to near the base of the anther (Figs. 11f; 12d; 13c). The interpretation by Cornet (1986) that the microsporophylls had two wall layers (i.e. epidermal and endothecial) was based on the preservation of a thick-walled cell layer surrounding the pollen masses and tapetum, and a thin outer layer with wrinkled cuticle (Fig. 11d, 11e). An epidermal cuticle was originally not verified because of poor preservation, but is verified here from the maceration of anthers belonging to the specimen in Figure 11c. Psilate monosulcate pollen was found abundantly clinging to one side of epidermal cuticles from this specimen (Fig. 13b), and is identical to that observed within the pollen chambers (Fig. 12b) or isolated from them (Fig. 13d-e).
The paired nature of the pollen sacs, their separation by a septum that disappeared with pollen maturity, and the lack of a true pollen sac wall were three aspects of Synangispadixis anthers strongly stressed by Cornet (1986). He gives several illustrations or drawings of anthers that purportedly show a septum separating two pollen masses, but no clearer documentation of this condition can be presented than sections through anthers. Figure 13c shows a portion of a male 'flower' that was embedded in plastic, sectioned, and polished for reflected light study. The position and orientation of the floral axis is indicated, and the section cuts anthers trans-longitudinally progressively higher from lower left to upper right. The ability of this section to traverse so many anthers in a sequential fashion indicates that they were borne in an orderly fashion (i.e. in rows). The pollen 'sacs' (P) appear as narrow elongate pollen masses with tapering ends, while the light reflecting ground mass represents anther wall material. The pollen masses are each composed of hundreds of compressed pollen grains (Fig. 13a, 13c) and occur in distinctive pairs (arrows) separated by septa (s). An isolated pollen mass is shown in Figure 13a for comparison. No morphologically distinct pollen sac wall or cuticle can be distinguished in most specimens. Pollen and cuticle preparations of anthers sometimes show a discontinuous cuticle preserved on the outside of parts of some pollen masses, suggesting the possible presence of a vestigial or incomplete pollen sac wall. The disappearance of the septum basally brings the pollen masses together (Fig. 13c, lower left), a condition noted by Cornet (1986: Fig. 4b). The appressed boundaries of individual anthers can be followed as faint dark lines (Fig. 13c, small arrows), while the 'ventral' suture dividing the anther into two symmetrical halves can be recognized near the base of one anther (Fig. 13c, open arrow). No known fossil or recent gymnosperm possesses this type of microsporophyll construction, and the additional paired condition of the anthers makes comparison with angiosperms the only reasonable choice.
General discussion
A special origin for a pollen tube transmission tissue rarely has been addressed in theories of carpel evolution in angiosperms (Cornet, 1986), and it is generally assumed to have evolved from carpellary (i.e. leaf) tissue when the 'need' arose (cf. Bailey and Swamy, 1951). The varying distribution of transmission tissue within the angiosperms relative to differences in ovule and stigma position, and its integrity even when reduced to a single cell layer of the ovary wall (e.g. Welk et al., 1965) suggest that it is a specialized organ requiring an individual or separate origin. The similarity in size, shape, and form of the Axelrodia middle-layer to sterile interseminal scales in the gnetalean-like ovuliferous 'flower' (Fig. 7d) suggests that they may be homologues. By homology the transmission tissue of angiosperms would also be derived from highly modified interseminal scales.
Doyle and Donoghue (1986, 1987a, b) and Crane (1985) provide cladistic evidence that the Bennettitales and Gnetales are sister groups of angiosperms, making a search for organ and character homologies with angiosperms a logical consequence. Harris (1932) and Crane (1985) interpret bennettitalean sterile interseminal scales as having developed from ovule primordia, making them equal to ovules but without nucellus and egg. Such an origin of transmission tissue from modified ovular integument would mean that the pollen tube in angiosperms could be following ovule-derived organs from stigma to micropyle (cf. Hill and Lord, 1987; Mulcahy and Mulcahy, 1987; Murdy and Carter, 1987). Such an origin would also mean that the expression of gametophytic self-incompatibility in the style, placenta, integuments, and micropyle (Zavada, 1984; Zavada and Taylor, 1986) is in reality different organ responses of the same developmental system. Synergid degeneration is known to be induced by pollination well before pollen tubes enter the ovary in some angiosperms (Mulcahy and Mulcahy, 1987), indicating a communication between stigma and embryo sac that could be accomplished only by a closed (i.e. unified) organ system. Consequently, if angiosperm transmission tissue is derived from homologues of bennettitalean interseminal scales, it would represent a further specialization of the ovulary system rather than a transfer of function to a leaf-derived organ with no 'prior experience'.
The presence of a middle layer with transmission tissue characteristics, which extends from an apical stigma to subbasal ovules in the Axelrodia carpel (Figs 9a-b; 6d, 6g) supports the importance of a unified self-incompatibility system in the evolution of angiospermy (Zavada, 1984; Zavada and Taylor, 1986), and suggests that carpel closure around unitegmic ovules, by itself, probably would not lead to angiospermy (a number of fossil and recent gymnosperms, such as Glossopteris, Caytonia, Hirmeriella and Araucaria, completely enclose their ovules with bract- or leaf-derived organs without developing a system like that of angiosperms; cf. Doyle and Donoghue, 1986). Ironically, carpel closure, considered by most botanists as the hallmark of angiospermy, may not be the cause of angiospermy (Zavada, 1984), because an intervening organ (i.e. interseminal scales) may have enclosed and shielded the ovules as in the Bennettitales when the carpel was still open (compare Fig. 7b and 7d). A massive type of transmission tissue in the Chloranthaceae that surrounds a solitary ovule and projects above the carpel through a short stylar canal to form a separate stigma in Hedyosmum (most obvious), Ascarina, Sarcandra, and Chloranthus (least obvious: Endress, 1987a) supports an early origin of that organ system. Complete closure of the Axelrodia carpel may have occurred as a secondary and additional control to reduce selfing (and flower abortion) by physically limiting the area of pollen germination, thereby increasing the chances for biotic cross-pollination with compatible pollen (Zavada, 1984, and references therein).
The bitegmic ovule is considered basic for angiosperms, while the unitegmic condition of some angiosperms is interpreted as derived (Crane, 1985; Doyle and Donoghue, 1986). The second or outer integument is not homologized with any pre-existing organ, and is considered to have evolved de novo in angiosperms (Doyle and Donoghue, 1986). The evolution of a transmission tissue carrying specific types of glandular cells that form a chemical pathway from stigma to micropyle may have reequired the evolution of a second integument with similar cellular (i.e. transmission-diffusion) properties as the transmission tissue in order for the ovule to assess male compatibility remotely (cf. Zavada, 1984; Zavada and Taylor, 1986). The second integument of angiosperms could be an outgrowth of transmission tissue, serving to maintain a unified organ system. Such an origin is indicated by its subepidermal or subovule origin (Eames, 1961), although the same evidence could be used to support an origin from surrounding interseminal scales (examine the morphology of the robust axial transmission tissue, obturator, orthotropous-ovule system in Anthurium [Araceae] for an example showing possible outgrowth).
Why paired pollen sacs are basic to angiosperm anthers and why the septum in angiosperms degenerates near anthesis are questions that bear directly on the origin of the angiosperm anther. Synangispadixis may provide some clues to answer these questions once correct homologies are made between angiosperm, gnetalean, and bennettitalean microsporophylls. The bivalved microsporophyll with a central cluster of free pollen sacs may be basic for the Gnetales (cf. Cornet, 1987a), even though later reduction may account for the absence of paired bracts in Welwitschia. The basic construction of bennettitalean microsporophylls is similar to that of Synangispadixis, except that more than two pollen sacs are fused to the adaxial surfaces of paired bracts and the bracts are not folded to enclose the sacs (Crane, 1985). In Synangispadixis space limitation and bilateral symmetry created by folding or conduplication of the bract would necessitate the reduction of median pollen sacs, raising the possibility that the septum is derived from modified sporogenous tissue and represents all that remains of the reduced sacs. Both the pollen sac 'wall' (i.e. tapetum) and septum degenerated with pollen maturity (Cornet, 1986), suggesting that they are derived from homologous organs. The absence of a true pollen sac waIl (as contrasted with the sporangium or anther wall) is considered by Eames (1961: p. 127) to be 'an important angiosperm character, one that will play a major part in the determination of the ancestry of angiosperms'.
Carpel and ovule ontogeny as indicators of angiospermy
The possession of a megasporophyll that morphologically and anatomically resembles a closed angiosperm carpel is the first major test of angiospermous affinity, but it tells us little about how the reproduction system functioned. A pollen-tube transmission tissue interconnecting ovules with stigma is an angiosperm character, suggesting male assessment by the female, but it tells us nothing about the gametophyte. Indirect evidence of nutrient rewards for insects by the presence of glands, and direct evidence of actual insect visitation in the form of bite marks and trails of selective anther debris on the carpel are important for understanding pollination biology, but are not evidence for angiospermy. The two sister groups of angiosperms, the Gnetales and Bennettitales, are either known to be or suspected of having been visited by and effectively pollinated by insects (Bino et al., 1984; Crepet and Friis, 1987). Thus, one of the most important aspects about Sanmiguelia reproduction - whether fertilization occurred before the ovule underwent significant development - must be documented either directly by an embryo at an early stage of ovule development or indirectly by carpel and ovule ontogeny.
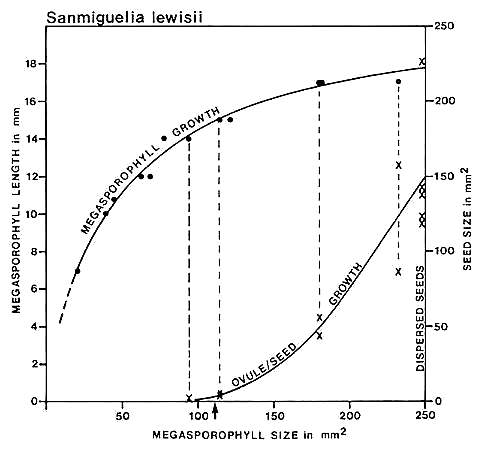
Fig. 14. Graph of carpel and ovule/seed growth for Sanmiguelia lewisii (Axelrodia) based on megasporophyll (carpel) length versus two-dimensional area: ovule/seed size or two-dimensional area is plotted on the ordinate under the points representing the carpels to which they belong; the curves were drawn by eye. Note the lack of ovule development below a certain minimal carpel size and length (14 mm), followed by the exponential growth of seeds to fill the ovary. Arrow marks estimated time of fertilization.
Cornet (1986) graphed carpel length against two-dimensional area and compared the resulting curve with the growth of enclosed ovules and dispersed seeds (Fig. 14). Difficulty in recognizing ovules in Axelrodia carpels smaller than 14 rnm in length, and their rapid (i.e. exponential) growth to fill the ovary in larger carpels suggested to him an angiospermous type of reproductive system. Furthermore, the morphology of embryo casts within dispersed seeds suggested that fertilization occurred before ovule development as in angiosperms. This interpretation, however, was limited by a lack of comparative data.
Carpel or megasporophyll and ovule/seed development were measured and plotted for three extant angiosperm species and one conifer. The resulting graphs for Magnolia grandiflora and M. virginiana (Fig. 15), Serelloa repens (Fig. 16) and Thuja orientalis (Fig. 17) compare carpel or cone length to weight measurement instead of two-dimensional size. This difference creates graphs that are not directly comparable with the graph for S. lewisii, but since two-dimensional compressions of originally hollow structures with thin walls expand when compressed, they are close to proportional to original size (i.e. surface area). The surface area of a hollow spheroid will increase as the spheroid increases in size, but its mass will not increase as rapidly as for a solid spheroid. As the thickness of the spheroid wall decreases, the change in mass will produce a curve that approaches the shape of the curve for change in surface area. That is probably why the graphs for Sanmiguelia and the angiosperms (particularly Magnolia) so closely resemble one another. Since the objective of these graphs is to document a delay in ovule development as in angiosperms, the data can be compared.
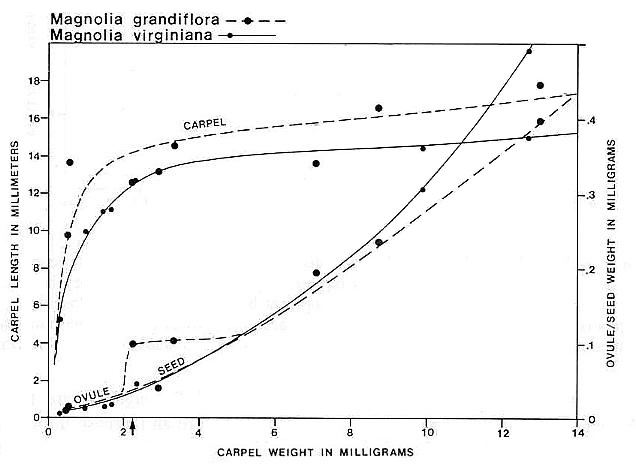
Fig. 15. Graph of carpel and ovule/seed growth for Magnolia grandiflora and M. virginiana (Magnoliaceae) based on carpel length versus weight in milligrams; ovule/seed weight is plotted on the ordinate under the points representing the carpels to which they belong; the curves were drawn by eye. Note the lack of ovule development until fertilization (represented by a certain minimal carpel size), followed by the exponential growth of seeds to fill the ovary. Arrow marks time of fertilization.
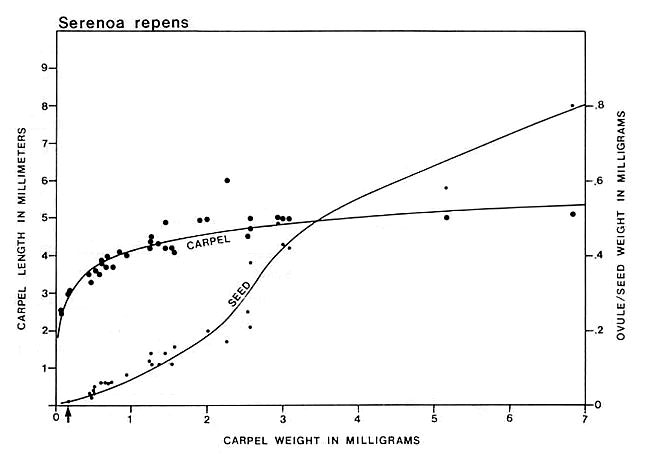
Fig. 16. Graph of carpel and ovule/seed growth for Serenoa repens (Arecaceae) based on carpel length versus weight in milligrams; ovule/seed weight is plotted on the ordinate under the points representing the carpels to which they belong; the curves were drawn by eye. Note the lack of ovule development until fertilization (represented by a certain minimal carpel size), followed by the exponential growth of seeds to fill thet ovary. Arrow marks time of fertilization.
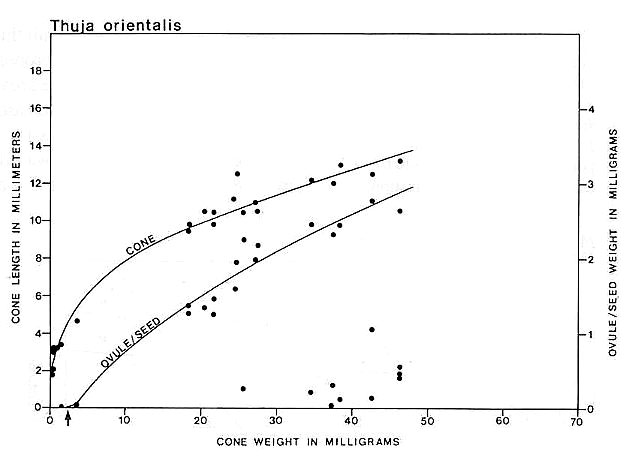
Fig. 17. Graph of cone and ovule/seed growth for Thuja orientalis based on cone length versus weight in milligrams; ovule/seed weight is plotted on the ordinate under the points representing the cones to which they belong; the curves were drawn by eye. Note that ovules are fertilized very early in development before cone scales enclose them, but seed development is unlike that in angiosperms or Sanmiguelia, because seed growth tracks the growth of the megasporophyll or cone; unfertilized or aborted ovules remain much smaller than the fertilized ones. Arrow marks time of fertilization.
The most significant comparisons between the angiosperms and Sanmiguelia are the rapid elongation of carpels before an increase in breadth or weight and the rapid or exponential growth of ovules only after a certain minimum length of carpel is reached. These similarities contrast with the development of Thuja megasporophyIls and ovules, which tend to track one another much more closely. Furthermore, there is no apparent delay in ovule growth as in angiosperms and Sanmiguelia. Thuja is pollinated very early before its cone scales grow over and conceal the ovules and their micropyles. In addition, delayed ovule growth in Sanmiguelia is much more pronounced than it is in Magnolia and Serenoa, which may be caused in part by the different methods of measurement. These differences, however, do not detract from the fact that Sanmiguelia ovules began showing rapid growth only after the carpel reached at least 14 mm in length and about 200 sq. mm in surface area (both sides of compression). The inflection points on carpel and ovule curves agree very closely with those for similar events in Magnolia and Serenoa, suggesting that ovule development did not proceed beyond a certain size unless fertilization occurred.
A delay in ovule development by itself is not necessarily evidence for angiospermy, because the delay could be an adaptation tied to compatible pollination (as opposed to compatible fertilization), reducing the female expenditure if an incompatible combination resulted in flower or carpel abortion (cf. Zavada, 1984; Zavada and Taylor, 1986). Such a delay in ovule development may have preceded the evolution of double fertilization and endosperm formation, since precocious fertilization could have had the effect of suppressing or circumventing nucellar and archegonial development. The development of a very large dicotyledonous embryo that filled the entire seed in Sanmiguelia (Cornet, 1986) suggests, however, that pre-fertilization nucellar food reserve, if it formed, did not occupy a significant volume of the ovule, otherwise it would have had an influence on embryo shape and size. Cornet (1986) was able to show that seed shape conformed very closely to the shape of the embryo, implying that both ovule and embryo growth were synchronous. Such a characteristic is found only in the angiosperms, and is a strong indication that the timing of ovule growth in Sanmiguelia was probably tied to fertilization.
Conclusions
Based on the works of Brown (1956), Tidwell et al. (1977), and Cornet (1986), the leaves, stems, roots, growth habit and habitat of Sanmiguelia lewisii indicate a semiaquatic woody herbaceous plant that resembled extant Veratrum californicum (the False Hellebore of the western United States) in form and stature, but which produced a series of clustered innovations of a few years' duration along a spreading rhizome. The rhizomatous habit, vesselless secondary xylem of restricted development in the lower part of the stem, and a dicotyledonous embryo (see Cornet, 1986, for documentation) are characters shared with Sarcandra and Chloranthus (Chloranthaceae), two of the most primitive living dicots (Carlquist, 1987). Both Sarcandra and Sanmiguelia have vessels restricted to their roots as in some monocots and ferns (Carlquist, 1987). The monocot-like leaf morphology and leaf venation, sheathing leaf base, and monocot-like inflorescences subtended by large spathe like vegetative leaves on a plant that otherwise compares with primitive herbaceous dicots indicate a plant more primitive than any living monocot or dicot.
 |
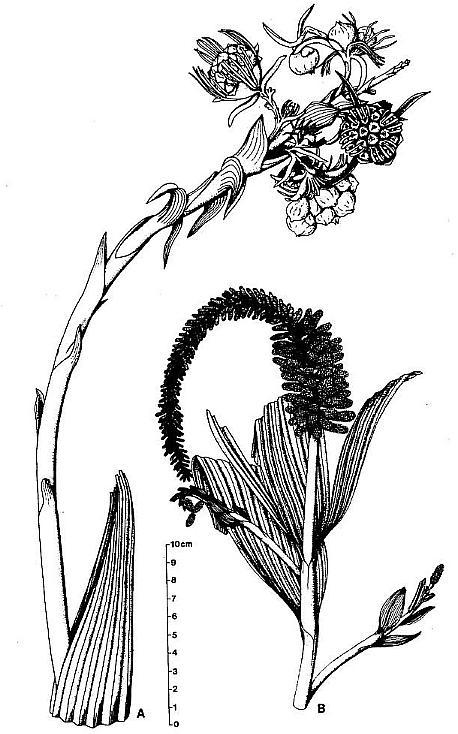 |
The presence of a closed carpel with ventral suture, an apical stigma, an ovarian organ probably adapted for pollen-tube transmission, paired subbasal anatropous ovules with indications of two integuments, ovules that probably developed only after fertilization, a dicotyledonous embryo with two large cotyledons, one of which was significantly larger than the other, ovuliferous flower-like units possessing a perianth comprised of large and specialized bracts, biloculate anthers borne in pairs and lacking distinct pollen sac walls, anther septa that disappeared at pollen maturity, and monosulcate pollen strongly indicate an angiospermous affinity. Clusters of female floral subunits borne separately below large apocarpous unisexual flowers, and male infloresences comprised solely of floral subunits that may be homologues of angiosperm stamen indicate a plant much more primitive than any angiosperm currently described from the Cretaceous (e.g. Retallack and Dilcher, 1981; Crane and Dilcher, 1984; Dilcher and Crane, 1984; Dilcher and Kovach, 1986; Friis and Crepet, 1987). Consequently, Sanmiguelia lewisii can be assigned only to the taxonomic level of Division (i.e. Angiospermae), and preserves an intermediate stage in the evolution of the angiosperm flower.
The early to middle Carnian Crinopolles Group of monocot-like pollen from the Richmond basin, Virginia (Cornet, 1989) demonstrates that numerous palynological characters thought to be typical and diagnostic of monocots (Doyle, 1913; Walker and Walker, 1984) appeared very early in the Mesozoic, possibly near the time of angiosperm origin (Crane, 1985; Doyle and Donoghue, 1986: p. 384, 1987b: p. 37). The presence of Sanmiguelia in the Carnian of North America supports Burger's (1981) contention that monocot characters played a significant role in early angiosperm morphology. Although there is still no clear evidence as to why angiosperms did not radiate in the Triassic or Jurassic, the fact that they did not can no longer be used as reasonable evidence for a later origin (Crane, 1988). Doyle and Donoghue (1987b: p. 41) suggest that carpel closure may be the reason for the radiation of angiosperms in the Cretaceous, but that cannot be the case if Sanmiguelia is an angiosperm or directly related to them. If double fertilization had not (yet) evolved in Sanmiguelia, carpel morphology and ovule development at least suggest the presence of an early self-incompatibilty system. The reason for a delayed radiation may therefore be more complex, having been the result of several factors including the evolution of deciduous leaves with petioles and hermaphroditic (i.e perfect) floral morphology; co-evolution with pollinators and the availability of numerous faithful pollinators; increased number of niches due to the extinctions of certain groups of gymnosperms; changes in climate, sea level, and available land masses; and a variety of factors associated with pollination, seed dispersal, and herbivory (Taylor, 1988). Perhaps, like Paleocene mammals, angiosperms had to wait for the right combination of changes in the Cretaceous (e.g. Bakker, 1978, 1986) before a sustained radiation could occur.
Acknowledgements
This paper is dedicated to my wife, Bonnie Lee Cornet, for her determined and heroic struggle against the ravages of a merciless cancer. The author is grateful to the Department of Geological Sciences, Columbia University and Dr G. Bayliss, President of GeoChem Labs. Inc., Houston, TX for providing laboratory space and equipment, and for the use of reflectance and light microscopes. He acknowledges US National Science Foundation Grant BSR 87-17707 to P.E. Olsen for support to the author. He thanks W.A. McHale (President of Accumin Analysis Inc., Houston, TX) and the former Gulf Research & Development Co. (now Chevron) for SEM work; Dr P.A. Murry and E.B. Cornet for field assistance at the Sunday Canyon locality; Dr J. Morgan (retired, Exxon), Dr C. Sancetta (Lamont), and Dr L.E. Stover (Exxon Co., USA) for the use of their microscopes; and Drs P.R. Crane, P .E. Olsen, D. Peteet, A. Raymond and C. Sancetta for their helpful comments, advice, and suggested improvements for this manuscript.
References
Armbruster, W.S., 1984. The role of resin in angiosperm pollination:
ecological and chemical considerations. American Journal of Botany, 71: 1149-1160.
Arnold, C.A., 1963. Cordaites-type foliage associated with palm-like plants from the Upper
Triassic of southwestern Colorado. Mashewari Commemorative Volume. Journal of the
Indian Botanical Society, 42A: 1-4.
Ash, S.R., 1976. Occurrence of the controversial plant fossil Sanmiguelia in the
Upper Triassic of Texas. Journal of Paleontology, 50: 799-804.
Ash, S.R., 1987a. The upper Triassic red bed flora of the Colorado Plateau, western United
States. Journal of the Arizona-Nevada Academy of Science, 22: 95-105.
Ash, S.R., 1987b. Growth. habit and systematics of the Upper Triassic plant Pelourdea
poleoensis, southwestern USA. Review of Palaeobotany and Palynology, 51: 37-49.
Bailey, I.V. and Swamy, B.Q.L., 1951. The conduplicate carpel of dicotyledons and its
initial trends of specialization. American Journal of Botany, 38: 373-379.
Bailey, I. V. and Swamy, B.G.L., 1954. Contribution to Plant Anatomy. Chronica
Botanica Co.: Waltham, Massachusetts.
Bakker, R. T ., 1978. Dinosaur feeding behavior and the origin of flowering plants. Nature,
274: 661-663.
Bakker, R. T ., 1986. How dinosaurs invented flowers. Natural History, 11: 30-38.
Becker, H.F., 1972. Sanmiguelia, an enigma compounded. Paläontographica, 138B:
181-185.
Bino, R.I., Dafni, A. and Meeuse, A.D.I., 1984. Entomophily in the dioecious gymnosperm Ephedra
aphylla Forsk. (= E. alte C.A. Mey.), with some notes on E. campylopoda C.A.
Mey. I. Aspects of the entomophilous syndrome. Proc. Koninklijke Nederlandse Akademie
van Wetenschappen, 87C: 1-13.
Bock, W., 1969. The American Triassic Flora and Global Distribution, 2-3.
Geological Centre of Research Series: North Wales, Pennsylvania, 406 pp.
Brown, R. W., 1956. Palm-like plants from the Dolores Formation (Triassic), southwestern
Colorado. United States Geological Survey Professional Paper, 274H: 205-209.
Burger, W. C., 1977. The Piperales and the monocots. Alternate hypotheses for the origin
of monocotyledonous flowers. Botanical Review, 43: 345-393.
Burger, W.C., 1981. Heresy Revised: the monocot theory of angiosperm origin. Evolutionary
Theory, 5: 189-225.
Canright, I.E., 1952. The comparative morphology and relationships of the Magnoliaceae. I.
Trends of specialization in the stamens. American Journal of Botany, 39: 484-497.
Carlquist, S., 1987. Presence of vessels in wood of Sarcandra (Chloranthaceae);
comments on vessel origins in angiosperms. American Journal of Botany, 74:
1765-1771.
Cornet, B.,
1986. The reproductive structures and leaf venation of a Late Triassic angiosperm, Sanmiguelia
lewisii. Evolutionary Theory, 7: 231-309.
Cornet, B., 1987a. An Ephedra-like plant with cupulate flowers from the Late
Triassic Dockum Group of Texas (Abs. 236). American Journal of Botany, 74: 679.
Cornet, B., 1987b. Further evidence for the reproductive morphology of Sanmiguelia
lewisii and its bearing on angiosperm ancestry (Abs: 237). American Journal of
Botany, 74: 680.
Cornet,
B., 1989. Late Triassic angiosperm-like pollen from the Richmond rift basin of
Virginia, USA. Paläontographica, 213B: 37-87.
Cornet, B., In prep. A new gnetalean plant with cupulate flowers from the Late Triassic
Dockum Group of Texas. For American Journal of Botany.
Crane, P.R. and Dilcher, D.L, 1984. Lesqueria: an early angiosperm fruiting
axis from the mid-Cretaceous. Annals of the Missouri Botanical Garden, 71: 384-402.
Crane, P.R., 1985. Phylogenetic analysis of seed plants and the origin of angiosperms. Annals
of the Missouri Botanical Garden, 72: 716-793.
Crane, P.R., 1988. Review of Cornet, B., The leaf venation and reproductive structures of
a Late Triassic angiosperm, Sanmiguelia lewisii. Taxon, 36: 778-779.
Crepet, W.L. and Friis, E.M., 1987. The evolution of insect pollination in angiosperms.
In: Friis, E.M., Chaloner, W.G. and Crane, P.R. (eds). The Origins of Angiospenns and
their Biological Consequences. Cambridge University Press: Cambridge, pp. 181-201.
Cresti, M.,. Keijzer, C.I., Tiezzi, A., Ciampolini, F. and Focardi, S., 1986. Stigma of Nicotiana:
ultrastructural and biochemical studies. American Journal of Botany, 73,
1713-1722. Dahlgren, R.M.T., Clifford, H.T. and Yeo, P.F., 1985. The Families of the
Monocotyledons, Structure, Evolution and Taxonomy. Springer-Verlag: New York, 520 pp.
Daugherty, L.H., 1941. The Upper Triassic flora of Arizona, with a discussion of its
geological occurrence. Contributions to Paleonology, 526. Carnegie Institute:
Washington, 108 pp.
Dilcher, D.L. and Crane, P.R., 1984. Archaeanthus: an early angiosperm from
the Cenomanian of the western interior of North America. Annals of the Missouri
Botanical Garden, 62: 590-620.
Dilcher, D.L. and Kovach, W.L., 1986. Early angiosperm reproduction: Caloda delevoryana
gen. et sp. nov., a new fructification from the Dakota formation (Cenomanian) of
Kansas. American Journal of Botany, 73: 1230-1237.
Doyle, J.A., 1973. The monocotyledons: their evolution and comparative biology. V.
Fossil evidence on the early evolution of the monocotyledons. Quarterly Review
of.Biology, 48: 399-413.
Doyle, J .A. and Donoghue, M.J., 1986. Seed plant phylogeny and the origin of
angiosperms: an experimental cladistic approach. The Botanical Review, 52: 321-431.
Doyle, J.A. and Donoghue, M.J., 1987a. Relationships of angiosperms and Gnetales: a
numerical cladistic analysis. In: Spicer, R.A. and Thomas, B.A. (eds), Systematic and
Taxonomic Approaches in Paleobotany, 31. Oxford University Press: Oxford, pp. l77-198.
Doyle, J.A. and Donoghue, M.J., 1987b. The origin ofangiosperms: a cladistic approach. In:
Friis, E.M., Chaloner, W.G. and Crane, P.R. (eds), The Origins of Angiosperms and
their Biological Consequences. Cambridge University Press: Cambridge, pp. 17-50.
Dunay, R.E. and Fisher, M.J., 1979. Palynology of the Dockum Group (Upper Triassic),
Texas, USA. Review of Palaeobotany and Palynology, 28, 61-92.
Eames, A.J., 1961. Morphology of the Angiosperms. McGraw-Hill Book Company: New
York, 518 pp.
Endress, P .K., 1987a. The Chloranthaceae: reproductive structures and phylogenetic
position. Bot. Jahrb. Syst., 109: 153-226.
Endress, P.K., 1987b. Floral phyllotaxis and floral evolution. Bot. Jahrb. Syst., 108:
417-438.
Fisher, M.J. and Dunay, R.E., 1984. Palynology of the Petrified Forest: Member of the
Chinle Formation (Upper Triassic), Arizona, USA. Pollen et Spores, 26: 241-283.
Friis, E.M., Crane, P.R. and Pedersen, K.R., 1986. Floral evidence for Cretaceous
chloranthoid angiosperms. Nature, 320: 163-164.
Friis, E.M. and Crepet, W.L., 1987. Time of appearance of floral features. In: Friis,
E.M., Chaloner, W.G. and Crane, P.R. (eds), The Origins of Angiosperms and their
Biological Consequences. Cambridge University Press: Cambridge, pp.145-179.
Gould, R.E. and Delevoryas, T., 1977. The biology of Glossopteris: evidence from
petrified seed-bearing and pollen-bearing organs. Alcheringa, 1: 387-399.
Harris, T .M., 1932. The fossil flora of Scoresby Sound East Greenland. Part 3.
Caytoniales and Bennettitales. Meddelelser om Gronland, 85: 133 pp.
Hill, J.P. and Lord, E.M., 1987. Dynamics of pollen tube growth in the wild radish. Raphanus
raphanistrum (Brassicaceae). II. Morphology, cytochemistry and ultrastructure of
transmitting tissues, and path of pollen tube growth. American Journal of Botany, 74:
988-997.
Mulcahy, G.B. and Mulcahy, D.L., 1987. Induced pollen tube directionality. American
Journal of Botany, 74: 1458-1459.
Murdy, W.H., and Carter, M.E.B., 1987. Regulation of the timing of pollen gennination by
the pistil in Talinum mengesii (Portulaceae). American Journal of Botany, 74:
1888-1892.
Retallack, G. and Dilcher, D.L., 1981. Early angiosperm reproduction: Prisca reynoldsii
gen. et sp. nov. from Mid-Cretaceous coastal deposits in Kansas, USA. Paläontographica,
179B: 103-137.
Stebbins, G.L., 1974. Flowering Plants, Evolution above the Species Level. The
Belknap Press of Harvard University Press, Cambridge, Massachusettes, 399 pp.
Taylor, T.N., 1988. The origin and evolution of angiosperms. Atlas of Science: Animal
and Plant Sciences: 47-50.
Tidwell, W.D., Simper, A.D. and Thayn, G.F., 1977. Additional information concerning the
controversial Triassic plant: Sanmiguelia. Paläontographica. 163B: 143-151.
Walker, J.W. and Walker, A.G., 1984. illtrastructure of Lower Cretaceous angiosperm pollen
and the origin and early evolution of flowering plants. Annals of the Missouri
Botanical Garden, 71: 464-521.
Welk, S.M., Millington, W.F. and Rosen, W.G., 1965. Chemotropic activity and the pathway
of the pollen tube in Lily. American Journal of Botany, 52: 774-781.
Zavada, M.S., 1984. The relation between pollen exine sculpturing and self-incompatibility
mechanisms. Plant Systematics and evolution, 147: 63-78.
Zavada, M.S. and Taylor, T.N., 1986. The role of self-incompatibility and sexual selection
in the gymnosperm-angiosperm transition: a hypothesis. American Naturalist. 128:
538-549.