Cornet, B., 1996. Flowering Plant Origin, Evolution & Phylogeny. In (eds.) Taylor, D.W. & Hickey L.J., Chapman & Hall, Chapter 3, p. 32-67.
A New Gnetophyte from the Late Carnian (Late Triassic) of Texas and its Bearing on the Origin of the Angiosperm Carpel and Stamen
Bruce Cornet
Over the last 100 years, theories on the origin of the angiosperms have shifted like the ebb and flow of the tide. But even the most recent ideas expressed at the AIBS symposium in Iowa (Angiosperm Origin, Early Evolution and Phylogeny) are not entirely new. The monocot, Amentiferae, gnetalean, and bennettitalean theories of angiosperm origin (Eames, 1961; Arber and Parkin, 1907, 1908) were proposed long before Burger (1977; 1981), Crane (1985), and Doyle and Donoghue (1986b) resurrected the anthophyte, monocot, and paleoherb theories in modified form (see also Tucker and Douglas, Chapter 7). Even concepts about the time of angiosperm origin have been cyclical, ranging from Permo-Triassic (Axelrod, 1952, 1961) to Early Cretaceous (Wolfe et al., 1975; Hughes, 1976; Hickey and Doyle, 1977) and back to Permo-Triassic (Comet, 1986, 1989b; Cornet and Habib, 1992; Doyle and Donoghue, 1986a; Martin et al., 1989; Martin et al., 1993).
Instead of bringing us closer to resolving the morphology and origin of the first angiosperms, the study of Cretaceous angiosperms has brought us almost full circle to the realization that the Cretaceous radiation may represent only the phylogenetic history of the extant angiosperm flora, and not the history of angiosperm origin. Cladistics has extended the angiophyte clade or angiosperm phylogenetic lineage back at least to the Late Triassic and has focused interest on angiophyte sister groups for evaluating character polarity and importance. Fossil Gnetales are much less known than the extinct Bennettitales. Therefore, the discovery of a possible pregnetalean plant that occupied the same habitat as the controversial Late Triassic Sanmiguelia lewisii is of critical importance in the search for angiosperm ancestors.
GNETOPHYTES AND GNETALES
The Gnetales have been interpreted as transitional between gymnosperms and angiosperms at least as far back as Arber and Parkin (1908) and Wettstein (1911). This intermediate status was emphasized by the segregation of Ephedra, Welwitschia, and Gnetum into the subdivision Chlamydospermae (from the Greek word meaning seeds with an envelope or "cloak"). Some authors have even compared the outer envelope of the ovule of the gnetophytes with the carpel or ovary wall in angiosperms (cf. Gifford and Foster, 1989). But the notion that the Gnetales are closely related to angiosperms also cycled through a period of disfavor, only to reemerge due to new evidence favoring a very close relationship with angiosperms (Doyle and Donoghue, 1986a, 1992). The reassesment of the phylogenetic position of the Gnetales was in large part due to the contributions of Martens (1971 and references therein) and Muhammad and Sattler (1982), along with the advent and results of cladistic analysis and molecular-based phylogenetic studies (e.g. Zimmer et al., 1989).
It was not until the similarities or synapomorphies (e.g., siphonogamy, tunica-corpus, lignin chemistry, reduced megaspore wall, and granular exine) among the Gnetales, Bennettitales, Pentoxylales, and angiosperms were qualified within cladograms that their true sister-group relationships became apparent (Crane, 1985;_Doyle and Donoghue, 1986a, 1,986b, 1992): There was difficulty still in recognizing homologies for the carpel, anther, and second integument in the Gnetales. For example, Crane (1985) failed to recognize a cupule homolog (i.e., second integument) in the Gnetales, but both Crane (1985) and Doyle and Donoghue (1986b) recognized cupules in the Bennettitales and Pentoxylales. The angiosperm carpel was iqterpreted along conventional lines as derived from a simple conduplicate leaf-like megasporophyll that enclosed two marginal rows of uniovulate cupules (phyllosporous origin; see Taylor and Kirchner, Chapter 6). The carpel wall, however, was not resolved with any particular structure in nonangiospermous anthophytes. Furthermore, little comparison was made of microsporophyll morphology within the anthophytes other than to point out the apparent differences.
The reproductive morphology of the new Welwitschia-like plant described below provides a unique look at plesiomorphic characters and organs that have become reduced (modified) or lost in extant Gnetales, thus making homologies with angiosperm reproductive organs either unrecognizable or unconvincing, particularly when viewed through the bias of the conduplicate carpel/laminar stamen theory. In addition, these new (or lost) characters require a broadening of our concept of the Gnetales by defining gnetophytes as including the subclade Gnetales, and not as the complete equivalent (homolog) of the Gnetales. In this regard, the Gnetales are considered here to be the crown group, just as angiosperms are considered to be the crown group within the angiophytes (Doyle and Donoghue, 1993).
Materials and Methods
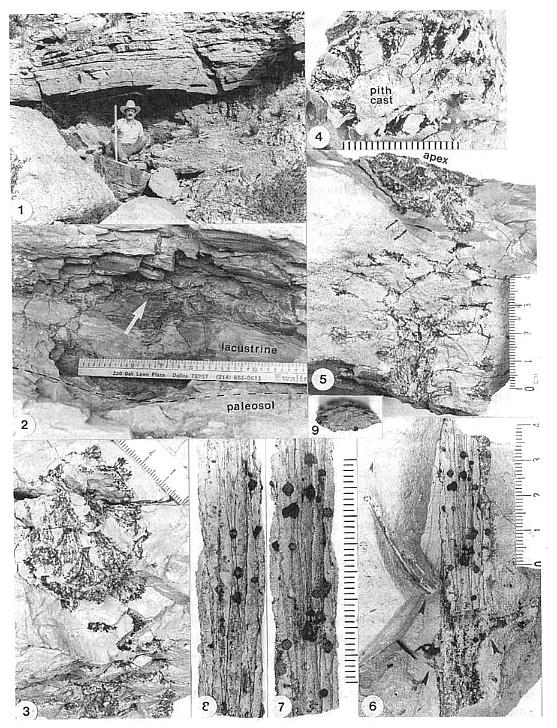
The holotype (female) for the genus was discovered on a field trip to Sunday Canyon in September, 1986. The paratype (male) and a second female reproductive axis were discovered in 1980 during the original excavation of the Sanmiguelia bed. All specimens come from one locality along a dirt road winding down the north wall of Sunday Canyon, just west of Palo Duro Canyon State Park, Randall County, Texas (latitude: 101o 44'; longitude: 34o 50'). The strata containing the plants occur just below a sequence of conglomerate and sandstone andjappear to represent a shallowing upward interdistributary lake deposit on top of a paleosol. The in situ plant bed, along with transported and dropped leaves, stems, and occasional allochthonous gymnosperm cones, is restricted to the west end of a long gray mudstone lens, which is terminated westward by a down-cutting sequence of channel sandstone (see Fig. 1 in Cornet, 1986). The new taxon was found in growth position above the siltstone paleosol (Figs. 3.1, and 3.2), and as fallen spikes within the paleosol, upon which a Sanmiguelia colony and ferns were found rooted [see Fig. 2 in Cornet, 1986) for a diagram of the plant bed; the holotype of the new taxon was found about 0.3 m behind the three vertical axes of Sanmiguelia connected by rhizomes shown to the left in that diagram].
Some of the specimens required degaging or breakage in order to reveal hidden parts. Most specimens, however, provided enough evidence for study and interpetation from the way they were initially exposed. Standard palynological techniques were usec! to secure pollen from microsporangia as well as cuticle fragments from reproductive organs. Preparations of pollen and cuticle were studied using a Zeiss binocular microscope and photographed with a Zeiss pbotomicroscope containing a built-in camera. Photographs of megafossils were made using a Minolta 35-mm camera with enlargement lenses and attachments.
The new reproductive organs are deposited at the Field Museum of Natural
History, Chicago, and include the following:
1. PP44195-a nearly complete female spike bearing close to 90
flowers, found above the paleosol oriented with apex pointed downward and base extending
into outcrop wall as if attached to a rooted plant. Excavation too dangerous to recover
more of specimen because of overburden. Specimen found in 1986 in first
pinkish siltstone layer above paleosol.
2. PP44196-a portion of a female spike found oriented
subvertically with pith cast uncompressed and flowers radiating outwards (undistorted).
Specimen found in 1980 in same pinkish siltstone layer containing PP4419S.
3. PP44197-a portion of the proximal sterile part of a spike
found lying horizontal at top of lacustrine mudstone lens to the east of main plant
bed and about 0.7 m higher in section. Specimen found in 1986 about
midway between western pinch-out of lacustrine unit and sharp bend in road as it turns
north up canyon wall.
4. PP44198-a collection of three male spikes found attached to a
common axis and bent around one another. Some portions of block containing specimens
not collected, because the size and extent of specimens was not apparent until prepared in
laboratory. Specimens found in 1980 within light gray siltstone paleosol, beneath in
situ Sanmiguelia colony and associated with isolated Pelourdea leaves, a log,
or large stem and,isolated conifer/pteridophyte male cones containing bisaccate pollen.
5. PP44199-microscope slides containing acid-resistant cuticles
and pollen from specimen PP44198.
Systematics
In this section the Welwitschia-like male and female reproductive axes are named and described as parts of a single species even though they were not found in organic attachInent. The similarity of the reproductive structures is so great as to leave little doubt that they represent the same plant; the occurrence of both male and female reproductive axes in the same layers is further support that they belong to the same taxon. It is unreasonable to expect organic attachment of these organs to the same plant if that plant were dioecious. Further justification of naming both types of organs as members of the same species is dependent on morphological comparison and will follow the taxonomic description in the discussion section. The two types of axes are described under separate diagnoses of the species. The term macrocupule is introduced for clarity, because Crane (1985) and Doyle and Donoghue (1986b) used the term cupule to include extraintegumentary structures of uncertain origin that surround the ovule. Macrocupule, as defmed here, is a much larger, cupule-like structure that was probably derived from one or more foliar bracts. The microsporangiate "flower" of Welwitschia mirabilis, for example, is interpreted here as a macrocupule.
Archaestrobilus Cornet gen. nov.
Type species. Archaestrobilus cupulanthus Cornet sp.
novo
Diagnosis. Unisexual macrocupules spirally borne on terminal
axes to form strobilus-like spikes. Female spikes at least 10 cm long; male spikes at
least 24 cm long. Macrocupules consist of a curled, bract-like organ with a narrow shaft
and flared funnel-shaped apex. Sutures represent convergent margins of bract; sutures
oriented apically (ventral). Female macrocupules contain one central elongate ovule or
seed with a bulbous base (bowling-pin shaped) and gradually tapering micropylar extension;
ovule surrounded by six to seven sterile scales (= interseminal scales when between
ovules) with expanded flat tops that terminate below apical rim of macrocupule and isolate
ovule from macrocupule wall. Ovules/seeds possess long, hair-like processes attached at
micropyle apex to form a parachute dispersal apparatus. Unusual structures with short
narrow pedicels and swollen heads are attached to outside of female macrocupule; these
structures larger and more numerous around the macrocupule rim, decreasing to small
surface omamenmtion along macrocupule shaft. Three to four short narrow bracts
surround base of female macrocupule. Male macrocupules without, central ovule or
vestigial ovule; contain thread-like appendages inside macrocupule instead of large
sterile scales. Hundreds of bivalved microsporophylls are densely packed along
length of shaft and around outside of funnel-shaped apex; no bracts present
on outside of male macrocupule. Microsporophylls consist of two ovate
bracteoles enclosing pollen sacs; three to five pollen sacs borne in cluster at end of narrow
axillary stalk. Bracteole attachment opposite at base of stalk. Pollen, simple,
monosulcate with thin subtectal granular-layer.
Click on images for enlargements.
Figures 3.1-3.9. 1. Sanmiguelia locality at Sunday Canyon, Texas, located
behind author; 3-ft. meterstick for scale; 9/86. 2. Close-up of lake bed and
paleosol containing in situ Sanmiguelia colony, Cladophlebis ferns, and Archaestrobilus;
location of holotype PP44195 shown; specimen found bent downward with apex embedded in
pinkish white siltstone/fine sandstone below, with base of strobilus projecting upward
(arrow, to left of specimen) into overlying gray claystone; axis then curved back down
toward paleosol; burial took place over an extended period of time; meterstick used for
scale; 9/86. 3. Archaestrobilus cupulanthus Cornet gen. et sp. novo (holotype
PP44195), female strobilus, apica1 view (same specimen as in Fig., 3.5); scale in
millimeters for this and all subsequent figures. 4. A. cupulanthus (PP44196),
female strobilus, showing large central pith as sediment-filled cast. 5. A.
cupulanthus (holotype PP44195), side view showing wide main axis with macrocupules
borne along its length. 6. Sterile base of reproductive axis (PP44197), bearing
three 3.8 cm long lanceolate bracts in a tight spiral, followed by long internode (arrows
point to bracts); pith cast in place, showing dark anastomosing and bifurcating grooves
filled with lignitic material representing probable traces of primary vascular bundles
that surrounded pith; isolated specimen found in same bed containing Archaestrobilus;
bracts unlike those of Axelrodia and Synangispadixis. 7-9. Close-up of
pith cast in Fig. 3.6, showing protoxylem traces, embedded in its outer surface,
anastomosing and bifurcating; round dark objects are hematite nodules (after pyrite?).
8. Opposite side from that shown in Fig. 3.7. 9. Cross-sectional view of pith
cast; elliptical shape due to compression.
Figures 3.10-3.15. 10. Pelourdea poleoensis leaf, basal part showing attachment to axis (inferred from contact) and portions of inferred clasping base torn away below stem; note only two sizes of parallel veins (one large, one very small), mostly dichotomous with no apparent cross-veins; specimen on same block with Archaestrobilus holotype (edge of PP44195 indicated). 11. Archaestrobilus cupulanthus (holotype PP44195), female strobilus, showing apical macrocupules removed to expose central axis (arrow); circle around macrocupule and attached bract (see Fig. 3.18 caption). 12. A. cupulanthus (holotype; detail from Fig. 3.3), close-up of apical macrocupules before removal (in Fig. 3.11), which are only partially compressed and still retain some three-dimensionality (upper left); axis below center of splay (axis shown in Fig. 3.11). 13. Detail from Fig. 3.12; close-up of partially three-dimensional macrocupules, showing ring of approximately seven sterile scales (B&W arrow heads) within funnel-shaped apex, and gland-like structures borne around rim of macrocupule (visible as black dots and fmger-like knobs along rim and perimeter; see Fig. 3.15 for detail); ventral suture (opening) to top of macrocupule indicated by arrow. 14. Three Archaestrobilus macrocupules in side view (holotype), showing flared apex, attachment to axis (below), and reduced (vestigial?) gland-like structures along shafts (small arrows). 15. Cross-sectional views of three different female macrocupules (vertical, linear black compressions with notched apices), showing open funnel-shaped apex with gland-like bodies attached, small bracts attached or positioned near base of macrocupules (large arrows), and reduced (vestigial?) giand-like structures along margin of shafts (small arrows).
Derivation. From archae - Greek, meaning beginning, first cause, old; strobilus - Latin, meaning twisted, spiral, pine-cone-like.
Archaestrobilus cupulanthus Cornet gen. et
sp. nov.
Holotype. PP44195, Figs. 3.2, 3.3, 3.5, 3.11-3.19, 3.21, and
3.22.
Paratype. PP44198, Fig. 3.4.
Type locality. Sunday Canyon, near Canyon, 'Texas, U.S.A.
Stratigraphic position. Trujillo Formation.
Age. Late Carnian (Late Triassic).
Derivation. From cupula - Latin for tub, vat, cup; anthos - Greek, meaning flower, in
reference to flower-like reproductive units possessed by various anthophytes.
Figures 3.16-3.22. 16. Archaestrobilus female macrocupules (holotype), showing apical, enlarged gland-like bodies around rim (darker elliptical to finger-like knobs, complete to partially exposed), cleft (ventral suture) becoming closed below rim (closed arrow), and tops of sterile scales near base of funnel-shaped apex (scales overlap and are not distinct from macrocupule except at top), represented in central specimen by carbonaceous compression material (open arrows). 17. Archaestrobilus female macrocupules (holotype), counterpart to specimens in Fig. 3.16 (detail from lower portion in Fig. 3.11), showing cleft in apical rim of central specimen (arrow), representing ventral suture. 18. Archaestrobilus female (holotype), showing bases of three macrocupules, central axis, and one exposed digitate bract (arrow), which is bent with one digit projecting downward; base of bract (labeled) normally constricted at point of attach- ment, and connected to an adjacent macrocupule not present on this specimen (but visible in Fig. 3.11; circled). 19. Enlargement of female macrocupule on right side of Fig. 3.18, showing very thin to open margins of macrocupule along side (small arrows; interpreted as the ventral suture), clay-flllings within sterile scales, portions of either deformed central cavity of macrocupule and/or ovule cast (labeled 0), which merges with suture traceable for entire length of that side (labeled S), and round nodule at base of macrocupule (large arrow). 20. Dispersed seed from Cow Branch Formation, Dan River/ Danville basin, North Carolina, lacustrine unit B12 (late Carnian; see Comet, 1993). 21. Seed cast (base) from macrocupule shown in Fig. 3.22; note longitudinal carbonaceous striations imbedded in surface of seed cast that represent either remnants of a corrugated seed coat or hair-like processes similar to those on seed in Fig. 20. 22. Archaestrobilus female macrocupule (holotype), showing seed cast in place with a gradually tapering apex (labeled apex) and curved base (small arrow, labeled base), and one digitate bract (labelled bract along one side) attached to outside of same macrocupule above base; note constriction of digitate bract at point of attachment, and finger-like apical margin (emphasized by curved lines just beyond bract tip).
Figures. 3.3-3.9 and 3.11-3.35.
Diagnostic description (female). Unisexual spikes borne
individually at end of main axis. Distal fertile part of spike up to 10 cm long, 5.8 cm
wide, containing about 100 spirally arranged macrocupules (Figs. 3.3 and 3.4). Spike axis
tapers from; 1.0 c~ proximally to 0.4 c91 di&tally. Sterile; basal pair of spike at
least 9 cm long, 14 mm wide (Fig. 3.6), bearing widely spaced, lanceolate bracts borne in
subopposite pairs (1 cm apart) separated by more than 7 cm; bracts 4-5 cm long and 4 mm
wide with parallel-veins. Large central pith (as a cast) in sterile base of spike extends
apically into lower, fertile part of spike (Fig. 3.4); compressed pith cast 11 mm wide in
sterile base (Figs. 3.7-3.9), possessing loose net of 15-17 anastomosing and bifurcating
protoxylem traces embedded in outer surface of pith cast. Macrocupules comprised of an
axially curled (tubular), bract-like organ with a narrow shaft and flared funnel-shaped
apex (Figs. 3.11-3.19). Sutures may represent convergent, unfused margins of the bract;
sutures oriented apically (ventral) along spike (Figs. 3.13-3.14, and 3.19). Macrocupules
2.0 cm long on distal part of spike, 2.3 cm long proximally; macrocupule shaft 3.2-5.0 mm
wide (4.5 mm average); flared funnel-shaped apex 5.0-7.0 mm long, 10.0-13.0 mm wide.
Macrocupules contain a single central ovule or seed with a bulbous base and a gradually
tapering elongate micropylar extension (bowling-pin shaped; Figs. 3.21 and 3.22); ovule
casts about 1.2 cm long, 2 mm wide at base, about 1 mm wide at apex (Figs. 3.21 and 3.22).
Ovules surrounded by six to seven sterile scales (= interseminal scales when between
ovules) with expanded, flat tops which terminate 3-4 mm below apical rim of macrocupule
and which isolate ovule from macrocupule wall (Fig. 3.13). Ovules/seeds possess long
hair-like processes attached at micropyle apex that form a parachute dispersal apparatus
(Figs. 3.20 and 3.21). Unusual structures with short narrow pedicels and swollen heads
attached to outside of female macrocupule (Figs. 3.11-3.17); these structures (0.6-1.1 mm
long; 0.4-0.5 mm wide heads) larger and more numerous around the macrocupule rim,
decreasing to small, scattered, clavate projections (0.2-0.4 mm long;, 0.1-0.2 mm heads)
albng macrocupule shaft (Figs. 3.14 and 3.15). Three to four 5 mm long digitate bracts
surround base of female macrocupule; bracts 0.8 mm at point of attachment to macrocupule,
widening rapidly to 2 mm with apical dissection into three to four short lobes (Figs. 3.18
and 3.22).
Figures 3.23-3.26. 23. Archaestrobilus cupulanthus (male; PP44198), top three male strobili attached to a common axis at X (basal attachement of axes faint in photographs; also strongly implied by orientation and position; the base strobilus No. 1 shown by arrow pointing in direction of apex. 24. A. cupulanthus (PP44198), with one piece of block removed to expose male strobilus No. 2, shown by arrows as it curves from left to right, with apex indicated by white arrow head, macrocupules mostly squashed laterally; scale in centimeters. 25. A. cupulanthus (PP44198}, with three pieces of block removed to expose strobilus No.3, shown by black arrows as it curves from right to left, with apex indicated by black arrow head; macrocupules mostly squashed proximal-distally; scale in centimeters. 26. Close-up of male macrocupules exposed in Fig. 3.24, belonging to male strobilus No. 2; note fragments of detached rnicrosporophylls dispersed in matrix between macrocupules (some of which are encircled; see transfer preparation in Fig. 3.37 for apparent density of dispersed microsporophylls).
Number of specimens examined. Two.
Diagnostic description (male). Unisexual spikes borne in
groups (of three) at the end of main axis (Figs. 3.23-3.25). Spikes fertile for almost
entire length from point of origin, with macrocupules spirally borne from apex down to
near base of spike. Spikes variable in length, ranging from 13 cm to 24 cm long (estimated
original length up to 33 cm long); spike width ranges from 5.2 cm to 6.3 cm for the
longest specimen. Central axis width decreases from 1.1 cm proximally to 0.6 cm distally.
Male macrocupules without proximal bracts (naked; Figs. 3.26 and 3-.27); without central
ovule or vestigial ovule. Filament-like appendages present inside macrocupule instead of
large sterile scales (Fig. 3.33). Ventral suture open on funnel-shaped apex, but
apparently closed (fused?) on shaft (Figs. 3.29-3.31). Macrocupule length relatively
uniform: about 2.2 cm long. Proximal shaft 1.5-3.0 mm wide; flared, funnel-shaped apex
6.0-9.0 mm long; 10.0-10.2 mm wide, increasing to 17-20 mm on macrocupules squashed
proximal-distally. Hundreds of bivalved microsporophylls densely packed along length of
shaft and around outside of funnel-shaped apex (Figs. 3.33 and 3.34). Microsporophylls
consist of two ovate bracteoles enclosing pollen sacs (Figs. 3.34; 3.37); bracteoles about
2.0 mm in diameter (Fig. 3.37). Three to five pollen sacs borne in cluster at end of
narrow axillary stalk (Figs 3.36-3.39); stalk 1.2 mm long, cylindrical to clavate with
constricted base; pollen sacs round to ovate, 0.3 km diam. to 0.3 mm; 0.5 mm; dehiscence
by longitudinal slit (Fig. 3.36): Bracteole attachment opposite at base of stalk. Pollen
simple, monosulcate with thin, subtectal, granular-layer (Figs. 3.40-41); pollen size
range: 19.2 mu x 9.6 mu to 30.4 mu x 16.0 mu (av.
length 24.5 mu; av. width 13.6 mu: 20 grains measured in four pollen
masses).
Number of specimens examined. Three.
Figures 3.27-3.35. 27. A. cupulanthus (male; PP44198); two male macrocupules of strobilus No. 2, one in side view (right) and one in cross section (left); note bivalved microsporophylls still attached on parts of rims (arrows) but missing along stalk; note also bracteoles and microsporophylls in matrix surrounding macrocupules (circled). 28. Counterpart to macrocupule on right in Fig. 3.27. 29. Funnel-shaped apex to a third male macrocupule from strobilus No. 2 (from between two blocks), viewed from its base (central sediment-filled cavity labeled core); note cleft (ventral suture; large arrow) open near rim; irregularity of cleft due to superimposed bracteoles (one of which is situated within cleft); cleft appears to be become a tear further down funnel-shaped apex where shaft begins, implying that cleft (suture) did not continue down shaft. 30. Funnel-shaped apices of two male macrocupules of strobilus No. 2 (PP44198); open arrows point to sutures aligned on same sides of macrocupules; alignment of sutures in ventral (apical) orientation. 31. Same two male macrocupules as in Fig. 3.30 viewed from below, showing orientation of ventral sutures; top suture spread apart by matrix; bottom suture closed with lips raised (open arrows); alignment of sutures in ventral (apical) orientation. 32. Three male macrocupules from strobilus No. 2 (PP44198), compressed laterally (arrows at bottom); attachment points of bivalved microsporophylls on macrocupule impressions appear as black (compression-filled) depressions where macrocupule wall pulled away, leaving microsporophyll bases exposed; scale same as Figs. 3.30 and 3.31. 33. Male macrocupules from strobilus No.3 (PP44198), squashed proximal-distally, showing thin thread-like sterile scales within central macrocupule (arrows), and concentration of numerous bracteoles to microsporophylls on outsides of macrocupules (three-dimensionall) extend into matrix; demonstrated to be outside on other specimens). 34. Oblique light view of raised (three-dimensionally preserved) bivalved microsporophylls around a proximo-distally compressed macrocupule in strobilus No. 3 (PP44198); note cluster of clam-shaped bracteoles and their crowded distribution whese they can be seen clearly (white arrow) due to shadow contrast. 35. Axial legion of male strobilus No. 2 (PP44198), near apex, showing bases of two male macrocupules (circled); wall of macrocupule can be seen as made up of carbonaceous, plate-like thickenings curved outward and distally from base of macrocupule (large arrow; details of base in right circle not as well preserved); within matrix-filled center of macrocupule base on left, a narrow, incomplete oval of black structures can be seen (small arrow within left circle), that may represent inner ring of thin thread-like sterile scales visible in Fig. 3.33.
DISCUSSION
The morphology of the reproductive organs, for the most part, is apparent and photogenic; however, some organs are quite different from comparable organs described in the literature (e.g., the microsporophylls), and model-dependent interpretation had to be avoided as much as possible. Some characters, although observed on a number of macrocupules, could be distinguished from artifacts of preservation only in one or two specimens having that character (e.g., suture) and showing no evidence of damage during transportation and burial. Reconstruction of some organs (e.g., pollen-sac apparatus) was dependent on several lines of evidence, anyone of which might by itself be equivocal because these organs are so different. Black-and-white photographs of organs were selected for their clarity and resolution of detail, but those goals were not always achieved, requiring additional labeling and notation on the photographs to highlight or encircle what is evident on the specimens.
Figures 3.36-3.42. 36. Archaestrobilus cupulanthus (male; PP44198) pollen-apparatus, recovered from basal portion of male strobilus No. 2 in Fig. 3.24; pollen apparatus consists of a central, inflated(?) stalk with a constricted base (a: 1.2 mm long) and round to oval pollen, sacs (b-d: 1.5-1.7 mm long) that were attached apically and possibly down one side of stalk (see Fig. 3.39); a few faint pollen grains (identified by morphology) appear within lowest pollen sac (d: long arrow); pollen-sac attachments are on right side and visible as nipples (short arrows); pollen-sac sutures longitudinal (aligned with long axis of sac) and difficult to see in photographs due to folds (suture on b along top margin; suture on c along bottom margin). 37. Transfer preparation of acid-resistant pollen-sac apparati (arrows) each encompassed by (overlapping) outlines of a pair of bracteoles (encircled; overlapping pairs most apparent in upper right circle); individual pollen-sac outlines can be discerned, but stalk obscured. 38. Pollen-sac apparatus exposed on surface of rock (white arrow: PP44198, strobilus No. 2), with at least four roundish pollen sacs visible in an arc around stalk (black arrow heads); stalk incomplete but black fragments of it are visible; pollen sacs to another apparatus exposed to right. 39. Transfer preparation of one pollen-sac apparatus, showing stalk with at least three small pollen sacs attached on left side (arrows); outline of bracteole on one side encircled (raised margin of bracteole cuticle just inside circle); slightly enlarged compared to Fig. 3.37. 40. Pollen mass (PP4419) recovered from bulk maceration of matrix from specimen PP44198; note monosulcate pollen around perimeter of mass; pollen ranges from 19 to 30 um in length; scale bar 20 um. 41. Pollen mass (PP44199), possibly from a small pollen sac, recovered from bulk maceration of matrix from specimen PP44198; same scale as Fig. 3.40. 42. Acid-resistant cuticle (PP44199) of bracteole belonging to bivalved microsporophyll, recovered from bulk maceration of mass from specimen PP44198; matrix came froth area containing abundant nilcrosporophyils (male strobilus No. 2), and only bracteoles and pollen-sac apparati have visible cuticle preserved under microscope examination.
The male and female spikes are constructed similarly, each possessing hundreds of spirally arranged, unisexual macrocupules of similar size (Fig. 3.43C). The reproductive axes are described as strobili or spikes and not as cones, because the reproductive units or macrocupules are radially symmetrical, not bilaterally flattened as in a cone. The male spikes tend to be longer than the female spikes and were apparently borne in clusters (three per cluster), whereas the female spikes were found as individual specimens (compare Figs. 3.3-3.5 and 3.23-3.26). Although the complete length of the spikes could not be determined, based on the reconstruction of the compound spikes in their blocks of matrix, the male axes were about three times the length of female axes. Unlike the unisexual reproductive organs of Sanmiguelia (from the same beds), which were also not found attached to the same specimen and which are quite different in morphology, the strobili of Archaestrobilus are very similar in overall construction, even down to corresponding details of the macrocupules. It is because of these similarities that they are described as a single natural taxon and given the same name.
Female Macrocupules
The macrocupules are radially symmetrical and trumpet shaped (Fig. 3.441). The presence of ventral sutures was determined from a few informative specimens where the sutures were spread apart or filled with sediment (Figs. 3.13 and 3.19, arrows). Their apical or ventral orientation (Fig. 3.43C) was determined by position relative to the central axis, and the occurrence of macrocupules were in succession with similarly oriented sutures. Such an orientation would be expected if the macrocupule is derived from a single bract.
Seeds. The morphology of the seeds was determined by seed casts, one of which was removed from its macrocupule (Eigs. 3.21 and 3.22). No nucellus or megaspore membrane is present. Thin, hair-like processes can be seen along the side of the seed. Similar and better preserved seeds were found associated with Pelourdea leaves in coeval strata of the Dan River basin, North Carolina (Fig. 3.20). The hairs appear to originate from the seed apex and flank the seed down to near its base (derived from an outer integument?).
Sterile scales. Based on the amount of compression material surrounding the ovule cast and the width of the macrocupule shafts, it is clear that tissue filled the space between the macrocupule wall and ovule (Figs. 3.13 and 3.16- 3.19). No cuticles are preserved, either on the outside of the macrocupules or within them. The presence of sterile scales surrounding the ovule is based on a few informative specimens at the apex of the spike that were only partially compressed (Fig. 3.13). In these obliquely distorted three-dimensional macrocupules, the tops of the sterile scales (pads) can be seen to form a floor near the base of the funnel-shaped apex (Figs. 3.13 and 3.16). The exact number of pads appears to be about six or seven, based on rings of scales that are clearly demarcated by borders with surrounding scales and structures (Fig. 3.13). The outer walls of the scales are preserved as carbonaceous compression material, whereas their centers decayed and became fIlled with clay (Figs. 3.13 and 2.19); distinction between clay filling spaces between scales and clay replacing cellular structure within scales was determined in longitudinal sections where carbonaceous compression material was observed capping or completing the apical outline of individual scales (e.g., Fig. 3.16). In longitudinal sections of macrocupules, the sterile scales appear to be contiguous with their neighbors and with the macrocupule wall (Figs. 3.18 and 3.19). Whether or not they were fused with the macrocupule wall or were freestanding could not be determined. The absence of cuticles that would distinguish individual structures could imply that they were joined or fused. The term "sterile" is used rather than "interseminal," because there is only one functional ovule. Clay typically replaces or fills the space occupied by the seed.
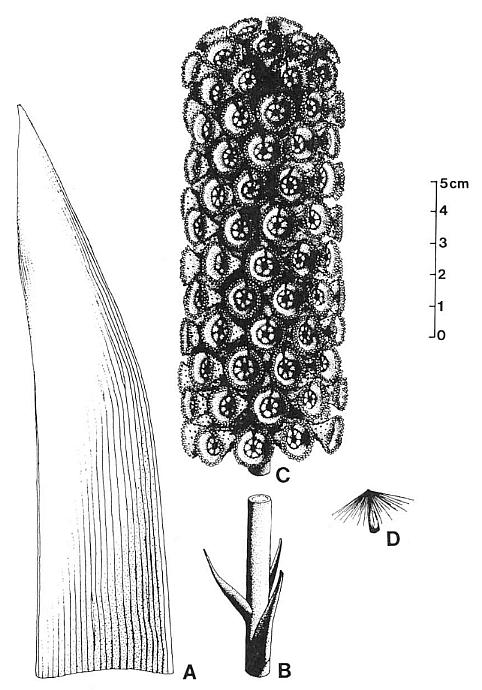
Figure 3.43. Reconstruction of Archaestrobilus cupulanthus and isolated organs related by morphology and association. (A) Associated leaf of Pelourdea poleoensis; most dichotomies occur near leaf base; cross-veins unknown. (B) Associated sterile lower part of strobilus. (C) Female strobilus (holotype). (D) Dispersed seed (Carnian, Dan River basin, NC) showing floater apparatus; same type of seed recovered from holotype.
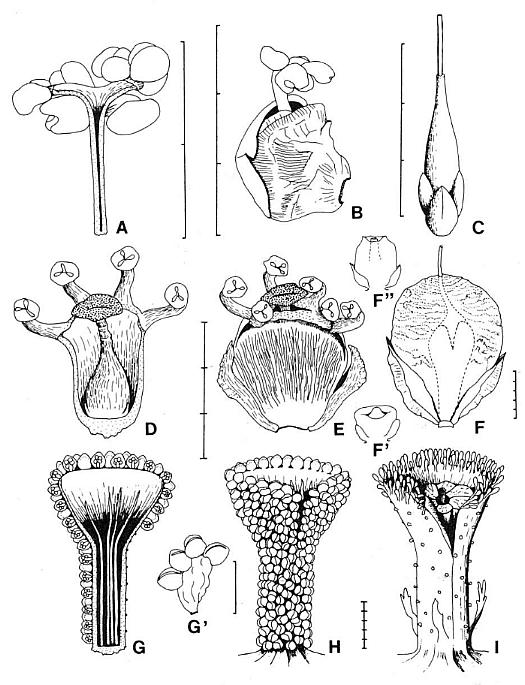
Figure 3.44. Comparison of gnetalean megasporangiate and microsporangiate organs with those of Archaestrobilus. (A) Cross section of Ephedra distycha male macrocupule. (B) Camera lucida drawing of E. distycha male macrocupule protruding from paired bracts. (C) E. trifurca, female macrocupule with basal pairs of opposite and decussate bracts. (D) Cross section of Welwitschia mirabilis male macrocupule (modified from Martens; 1971). (E) Drawing of W. mirabilis male macrocupule enclosed by basal pairs of opposite and decussate bracts (modified from Martens, 1971). (F) Drawing of W. mirabilis female macrocupule ("wing integument") and basal pair of opposite bracts (modified from Martens, 1971). (F'). Immature female macrocupule showing origin from a closed ring structure and not from a pair of fused bracts (after Martens, 1971). (F"). Immature female macrocupule further along in development, showing cupular origin of "wing integument." (G-H) Male macrocupule of Archaestrobilus cupulanthus. (G) Cross section showing reduced sterile (interseminal) scales inside, and bivalved microsporophylls outside macrocupule. (G') Pollen sac unit showing four pollen sacs attached to inflated stalk. (H) Outside view of male macrocupule showing suture open only on flared apex and crowded distribution of bivalved microsporophylls. (I) Female macrocupule of A. cupulanthus showing gland/food body-like organs crowded around rim (homologous organs reduced in size along below rim), suture peeled back to expose ring of sterile scales surrounding a central ovule with extended micropyle, and several digitate bracts attached around the base of the macrocupule.
Glands/Food Bodies. The unusual structures attached to the outside of the macrocupule may have functioned as glands and/or food bodies; they increase in size around the funnel-shaped apex, reaching their largest size around the macrocupule rim (compare Figs. 3.14 and 3.15 with Figs. 3.16 and 3.17). Their morphology on the rim is similar to the clavate stalks that support pollen sacs (Figs. 3.36 and 3.39), but they are not enclosed by a pair of bracteoles (Fig. 3.37).
Bracts. The bracts attached near the base of the macrocupules are difficult to demonstrate because of their small and delicate na:fure. The only two that were uncovered in their entirety are spatulate with a digitate apex (Figs. 3.18 and 3.22). Other bracts were viewed in cross section (Fig. 3.15). The number per macrocupule (three to four) was based on the size and apparent spacing of the bracts, as no more than two could be clearly seen on anyone macrocupule, and they were not attached opposite one another as they should have been if paired.
Male Macrocupules
The male macrocupule-like the female ones, are radially symmetrical and trumpet shaped (Fig. 3.44H). The shafts to the macrocupules are narrower than the shafts of female macrocupules due to the absence of a central ovule. The presence of ventral sutures was determined from a few informative specimens where the sutures were spread apart and the space filled with sediment (Figs. 3.29-3.31). Their apical or ventral orientation was determined by their position relative to the central axis. Below the funnel-shaped apex, crowded microsporophylls obscure the suture (Fig. 3.44H). Macrocupules compressed proximo-distally indicate that the suture was probably closed along the shaft, because it separated (tore?) only on the funnel-shaped apex (Fig. 3.29).
Sterile Scales. Equivalents or homologs to the sterile scales of the female macrocupules appear to be present within the male macrocupules. Their narrow filament-like morphology is apparent in only a few macrocupules that were squashed proximo-distally (Fig. 3.33). A partial ring of these scales is visible at the base of one macrocupule (Fig. 3.35). The number of such processes or sterile scales could not be determined. They are distinct from tracheids or tracheid bundles by their much larger size (i.e., width), color (tracheids appear black, whereas the scales appear as light-colored impressions sometimes with a dark vascular trace in the middle), and position within a hollow macrocupule.
Bivalved Microsporophylls. Crowded clam-shell-shaped microsporophylls attached to the outside of the male macrocupule are similar in size from macrocupule base to apex (Figs. 3.34 and 3.44H). The ones around the macrocupule apex and rim appear to be more stout or better attached, because they persist even when microsporophylls along the shaft have been removed by abrasion during burial or by insect herbivory. In specimens in which the microsporophylls are not attached, bracteoles can be seen distributed in the matrix between the macrocupules (Figs. 3.26, 3.27, 3.30 and 3.31). Bracteole cuticle was recovered that shows the faint outline of epidermal cells (Fig. 3.42).
Pollen-sac apparatus. Pollen sacs are attached to the ends of cylindrical to club-shaped axes or stalks (Figs. 3.36-3.39; 3.44G, and 3.44G'). A few specimens were observed on broken surfaces or in transfer preparations where three to four pollen sacs were still attached or arranged distally on the stalk (Figs. 3.37 and 3.38). The size of the pollen-sac apparatus is very small and, unfortunately, was too fragile to be recovered intact. In transfer preparations, pollen- sac apparati were found within the concavities formed by a pair of bracteoles (which are outlined by their acid-resistant cuticles) and situated opposite their adaxial sides (Figs. 3.37 and 3.39), but only one specimen clearly shows (immature?) pollen sacs situated around the apex of the central stalk (Fig. 3.39). Maceration produced numerous specimens of stalks and pollen sacs, but none attached to one another (Fig. 3.36). Attachment scars on the pollen Sacs were detected, along with torn margins on the stalks, confirming observations with the binocular microscope. The stalk cuticle is similar to that of the pollen sacs, implying a common derivation (Fig. 3.36), perhaps from a modified pollen sac. Carbonaceous material frequently coats the outside of the stalk cuticle, whereas no carbonaceous residue was found inside the compressed cuticle envelope (compare Figs. 3.36 and 3.39). The bulging and irregular, folded sides of the stalk cuticle and absence of internal residue suggest that the stalk was hollow and possibly inflated at pollen maturity. Inflation or elongation may have been necessary to elevate the pollen sacs above the bracteoles. One side of the stalk appears to be incomplete on all recovered specimens, implying either that the stalk is inverted or that pollen sacs were attached down one side (Figs. 3.36 and 3.44G').
Pollen. Pollen masses were recovered in bulk maceration of microsporophylls. The size and shape of these masses (Figs. 3.40 and 3.41) is identical to the size and shape of the pollen sacs. Only one type of pollen is present in these masses: simple, monosulcate with a thin translucent wall (Figs. 3.40 and 3.41) and infratectal granules, which are barely visible as tiny beads within some specimens. Some pollen sacs still contain this type of pollen (Fig. 3.36, arrow), but poor contrast makes the pollen difficult to see in photographs.
Strobilus Base. The sterile basal part of the female axis (Figs. 3.6-3.9) was not found attached, but based on its morphology, size, and pith cast, it is thought to belong to Archaestrobilus (Fig. 3.43B). The three long narrow lanceolate bracts on this specimen are unlike the short bracts borne by Axelrodia, or the leaves of Sanmiguelia, the only other seed plant found preserved in situ at this locality. One bract bears the impressions of a larger central vein flanked by several small, parallel veins (barely visible in Fig. 3.6). The close spacing of the bracts in a tight spiral, followed by a long internode (Fig. 3.43B), is similar to the manner in which the male spikes are borne in what appears to be a trichotomy of determinate branches. It cannot be determined whether the sterile base represents the lower part of the compound male strobili or the base of the solitary female spike, In either case, its morphology is distinctive and its bracts similar to the leaves of Pelourdea, which also occurs as in situ specimens (Fig. 3.10).
INTERPRETATION
Biotic Implications
The close similarity in morphology between male and female, macrocupules implies that they are homologous. The presence of microsporophylls on the outside (male) and an ovule on the inside (female) implies a derivation from a bisexual ancestor (cf. Welwitschia). The similarity in morphology and position of the pedicellate structures on the outside of the female macrocupule to the stalks of microsporophylls also implies homology. The presence of such structures on the female macrocupules indicates that they may have had a function in attracting insects looking for food or pollen. The carbonaceous material coating the outside of the pollen-sac stalks of the male macrocupules has a resinous look about it, as do the swollen heads of similar but reduced structures around the rim of the female macrocupule. No chemical analysis of these organs has been made, however. The concentration of modified appendages around the rim of the female macrocupule is probably a modification to enhance insect attraction to the tops of macrocupules where the micropyle was exposed. These observations are the basis for interpreting the sterile organs on the outside of female macrocupules as glandular food bodies. The swollen heads of the sterile scales around the ovule may have provided a convenient landing pad for insects and a place for them to stand while feeding on the surrounding garden of possible food bodies.
The dense aggregation of bivalved microsporophylls around the entire outer surface of the male macrocupules would be difficult to explain in an anemophilous plant. On the one hand, the close spacing of the macrocupules on the axis would prevent wind from having access to pollen released below the apical rim. The paired bracteoles are unusual for an anemophilous plant, in that they would baffle effective wind velocities between the macrocupules. On the other hand, the bracteoles may have served to protect the pollen from insect herbivory until maturity. The spaces between the macrocupules were wide enough to allow small beetles, for example, to live and forage there. The bracteoles and pollen sacs on one male spike were largely removed from below the rims of most of the macrocupules, whereas on another, they remain intact. The removed bracteoles occur disseminated through the matrix between the macrocupules, and torn attachment points can be seen on macrocupule shafts. Perhaps insect foraging is responsible. Wind was probably effective in dispersing pollen from sacs around the rim, but copious amounts of pollen that had to have been produced by such large spikes coud only be justified as an efficient use of energy if insects were the prime pollinators. Thus, Archaestrobilus cupulanthus is interpreted as ambophilous.
Association with Pelourdea
At the Sunday Canyon locality isolated leaves of Pelourdea poleoensis were found in the paleosol underlying the Sanmiguelia root zone and in thin shales interbedded within the sandstone sequence overlying the Sanmiguelia colony (Cornet, 1989). A long strap-shaped leaf with poorly preserved parallel venation matching the overall morphology of Pelourdea was found next to the blocks of paleosol containing the male spikes. Leaves of both taxa are frequently reported from the same localities (Ash, 1987). On the back side of the holotype (female spike: PP44195) occurs a portion of a stem with an attached fragment of a (clasping) leaf base and connected lower part of a well preserved P. poleoensis leaf (Fig. 3.10). This leaf fragment shows the typical crowded parallel venation of P. poleoensis described by Ash (1987), as well as minor second-order venation between the primary veins. Bifurcations are more numerous than anastomoses, but subperpendicular cross veins are lacking. The apparent absence of cross veins on siltstone impressions could be the result of preservation (Daugherty, 1941; Stagner, 1941; Ash, 1987), but this specimen is preserved well enough to show that cross-veins are not present in the lower part of the leaf.
Even though this stem is not oriented in the same direction as the reclining spike, the association of Pelourdea leaves with Archaestrobilus, in the absence of any other in situ candidates, implies a relationship. That is why a Pelourdea leaf is shown next to Archaestrobilus in Fig. 3.43.
Affinity
The presence of a single ovule at the center of unisexual macrocupules, which appear to be arise from the axil of a single bract, and the presence of male macrocupules bearing stalked clusters of three to four (possibly six) pollen sacs (Figs. 3.44G-I and Fig. 3.45F) are strongly reminiscent of the Gnetales, particularly Welwitschta (Fig. 3.44A-F; cf. Martens, 1971; 1977; Crane, 1985; Crane, 1988b; Gifford and Foster, 1989). Other possible synapomorphies with the Gnetales include the following: elongate parallel-veined leaves/bracts (cf. Welwitschia, if Archaestrobilus belongs to Pelourdea); a distinctive pith surrounded by a loose net of anastomosing and bifurcating protoxylem bundles in the inflorescence axis (this may also be a plesiomorphic character for the anthophytes); ancestral bisexual macrocupules (cf. Welwitschia: Fig. 3.44D); sterile scales surrounding the ovule (cf. rudimentary appendages surrounding ovule in the male macrocupule of Welwitschia: Fig. 3.44D; Martens, 1971); male strobili borne in clusters of three (i.e., compound strobili; cf. Welwitschia); and monosulcate pollen with optical indications of infratectal granules (cf. Welwitschia). The fact that the bracts on the sterile base of the spike(s) are borne in a tight spiral of three may be significant, inasmuch as this phyllotaxis is intermediate between spiral and whorled/paired (a synapomorphy for extant Gnetales). Clearly, the greatest overall similarity is with Welwitschia.
The spiral arrangement of macrocupules on the reproductive axes (Fig. 3.43C), radial symmetry of both male and female macrocupules (Fig. 3.44H-I), sterile scales or bracteoles that surround a central ovule (Fig. 3:44I), unisexual macrocupules, sterile, bractless microsporophylls (glands/food bodies: interpretation) on the outside of the female macrocupule (Fig. 3.441), spiral arrangement of determinate leaves on the stem (if Archaestrobilus belongs to Pelourdea), parallel leaf and bract venation with few cross viens (Fig. 3.10), simple monosulcate pollen with no ribs or plications, and a large pith with anastomosing and bifurcating protoxylem bundles may all be plesiomorphic characters, indicating that Archaestrobilus is much more primitive than the extant Gnetales. Its pollen most closely resembles that of Sahnia laxiphora (Pentoxylales) under transmitted light (Osborn et al., 1991), consistent with an anthophyte affinity. However, the cupular morphology of its reproductive organs, the presence of only one functional ovule per macrocupule, pollen sacs clustered together (not synangia - free from enclosing bracteoles), and compound strobili may be the most important characters for determining its affinity.
I therefore suggest that Archaestrobilus cupulanthus is a stem gnetophyte, because of the number of characters which unite it with the Gnetales and which distinguish gnetophytes from all other anthophytes. The absence of decussate and opposite leaves cuuently excludes Archaestrobilus from the Gnetales but not from the gnetophytes, which are more broadly defined here by the recognition and circumscription of Archaestrobilus. Consequently, inclusion of Archaestrobilus and any other non-gnetalean seed plant in the gnetophytes is based mostly on reproductive rather than vegetative similarity, just as the inclusion of Sanmiguelia in the angiophytes is based on its overall suite of characters, not on its having all the characters (e.g., leaf morphology) of Cretaceous or extant angiosperms.
Homologies among Archaestrobilus, Ephedra, and Welwitschia
According to Crane (1985) and Doyle and Donoghue (1986a, 1986b, 1992; Hickey and Taylor, Chapter 8), Ephedra is the sister taxon to Welwitschia plus Gnetum, because it has the most plesiomorphic characters. The advances shared between Welwitschia and Gnetum over Ephedra are vein anastomoses in the leaves, reduction of the male gametophyte, a tetrasporic megagametophyte with - free nuclei serving as eggs, and a feeder in the embryo (Doyle and Donoghue, 1986a). The presence of double fertilization in Ephedra (Friedman, 1990a, 1990b) could also represent a plesiomorphic character for the anthophytes. Archaestrobilus possesses characters that may be plesiomorphic even. for Ephedra, such as radially symmetric floral units that are spirally arranged rather than decussate or whorled, and bracts that are grouped but still spirally arranged rather than opposite or whorled. The grouping of male spikes may be similar to that of the bracts in being arranged in a tight spiral, indicating that the characteristic synapomorphy for the Gnetales of opposite or whorled appendages is probably an advancement over the condition in stem gnetophytes (Doyle and Donoghue, 1986b).
Homologies among floral structures have been confused by misinterpretation and the previous absence of fossils that could clarify homologies among extant Gnetales. The structure enclosing the ovule in Welwitschia is a prime example: The wing or "aile" has been variously interpreted (Martens, 1971, p. 137), with Martens concluding that the wing consists of a pair of fused bracts. That interpretation was accepted by Crane (1985, 1988b). It probably stems from the observation that the male flower has two pairs of alternating and decussate bracts, the innermost pair of which most closely resembles the wing around the ovule on the female flower. Yet the data and illustrations in Martens (1971) contradict his own interpretation. Nowhere does a cross section or illustration of an immature female flower show two separate bracts (e.g., Martens, 1971, Figs. 66, 67, and 69). Only the diagramatic cross section (his interpretation) in his Fig. 63-6 shows two bracts. In all other cases, the actual specimens show a macrocupule or ring structure that grows upward to enclose the central ovule. Similarly, the outer envelope or "perianth" of the ovule in Ephedra was interpreted as arising from the fusion of a dorsal-ventral pair of bracts by Crane (1985, p. 759). That interpretation may stem from misinterpretation of Martens' (1971) floral diagrams for Ephedra distachya, Even when ovules are borne in pairs, each contains a single ring primordium that surrounds the ovule (Martens, 1971, Fig. 21).
If there is any indication of two separate bracts in the morphology of the ovuliferous wing of Welwitschia, the division between bracts would have to be placed in a dorsi-ventral position where the macrocupule wall is thinnest and its apical margin slightly cleft on the ventral side [contrary to floral diagrams in Martens (1971) and Crane (1985)]. That separation, which was suggested by Chamberlain (1966, Fig. 374B), would create two pairs of nonalternating conduplicate bracts - a symmetrical paradox. Here is a problem where a fossil can be used to distinguish between homology and analogous symmetry caused by bilateral (dorsi-ventral) flattening of the flower.
The existence of a tubular, or ring macrocupule in the Gnetales is well demonstrated by the male flower of Welwitschia (Fig. 3.44D-E). Immature female flowers of Welwitschia also show a tubular macrocupule before much dorsi-ventral flattening has occurred (Fig. 3.44F'-F"}. The outer envelope of the Ephedra ovule is also macrocupular in construction (Fig. 3,44C; Chamberlain, 1966; Gifford and Foster, 198.9). The male flower of Ephedra consists of a miniature macrocupule, enclosed by a pair of opposite bracts (Fig. 3.44A-B). Based on a reexamination of the male flowers of E. distachya, I discovered that the male macrocupule bears up to six pairs of pollen sacs (rarely three sacs per cluster) on and below the rim of a flared but dorsi-ventrally flattened funnel-shaped apex, with a central opening that becomes mostly occluded below the base of the funnel at the center of the shaft. Such morphology indicates a possible derivation from a hollow, radially symmetrical macrocupule, like that of Welwitschia and Archaestrobilus. The major difference between the male macrocupules of Welwitschia and Ephedra and those of Archaestrobilus is one of size (see Fig. 3.44).
Size reduction may account for the decrease in number of microsporophylls from hundreds in Archaestrobilus to as few as six in Welwitschia, and a similar decrease in the number of pollen sacs per microsporophyll from four to six in Archaestrobilus to two in Ephedra and Gnetum. Even with extreme size reduction, the pollen sac clusters remained stalked [this is not necessarily the case for all fossils of possible gnetalean affmity, e.g., Piroconites (van Konijnen- burg-van Cittert, 1992)]. Size reduction and enclosure by strobiloid bracts may also account for the loss of bracteoles that enclose the pollen sacs of Archaestrobilus (they become redundant), as well as the loss of microsporangia from much of the shaft in Ephedra and all of the shaft in Welwitschia. The presence of bracts subtending the male macrocupules in Ephedra and Welwitschia may be an apomorphy (transposition from the female macrocupule) if the naked condition in Archaestrobilus is basic. Alternatively it may indicate separate derivation of the Ephedra and Welwitschia lineage(s) from a common ancestry with Archaestrobilus, which still had functional bisporangiate macrocupules. That possibility would make Archaestrobilus the sister taxon of Ephedra plus Welwitschia. The macrocupules of Archaestrobilus obviously became dimorphic and unisexual very early in the evolution of the gnetophyte clade.
The microsporangiate structures (anthers sensu Martens, 1971) of Gnetum are much simpler in construction than a ring of pollen sacs borne on reduced macrocupules and enclosed by paired bracts that occurs in Ephedra and Welwitschia (Chamberlain, 1966; Martens, 1971). They consist of a narrow stalk bearing two terminal pollen sacs; this structure is enclosed by a tubular perianth with an apical slit or suture. On the one hand, if this tubular perianth were derived from the fusion of paired bracteoles like those of Archaestrobilus. Gnetum would be the only extant member of the family to retain vestiges of such bracteoles. On the other hand, if these structures are highly reduced versions of the macrocupules plus paired bracts of Ephedra and Welwitschia, the homology would be at a higher structural level. In Gnetum gnemon, aborted ovules are borne in a whorl around a central axis above the microsporophylls. If the outer envelope or tubular perianth that encloses the ovules is homologous with the macrocupule of Archaestrobilus and the microsporangiate structures are only slightly modified versions of bivalved microsporophylls, the only major differences from Archaestrobilus would be the restriction of microsporophylls to the bases of macrocupules in Gnetum and the whorled arrangement of those macrocupules.
Homologies Among Anthophyte Androecia
Cladistic analyses based either on phenotypic or on genotypic characters have been inconsistent and; therefore, inconclusive about interrelationships between anthophyte sister groups (cf. Crane, 1985; Doyle and Donoghue, 1986a, 1986b; Zimmer et al., 1989). But one fact remains consistently clear: The Bennettitales, Gnetales, Pentoxylales, and angiosperms had a common ancestry sometime before the Late Triassic. The Gnetales are relatives of angiosperms and Bennettitales that underwent drastic floral reduction and aggregation in response to wind-pollination (Doyle and Donoghue, 1986a). The number of similarities or synapomorphies (plesiomorphies for the group) involve nonpreservable characters: siphonogamy, tunica-corpus, lignin chemistry, reduced megaspore wall, and granular exine. Additional characters that unite the Bennettitales and Gnetales are stalked or sessile ovules, whorled microsporophylls, and a micropylar tube. The three living genera of Gnetales share opposite leaves (originally probably spirally arranged and linear), multiple axillary buds, vessels, loss of scalariform pitting, simple or reduced microsporophylls, a single terminal ovule, a second integument apparently derived from the macrocupule of the ovulate flower, and striate pollen (lost? in Gnetum: Doyle and Donoghue, 1986a).
To this list of characters which unite the anthophytes, in particular the Gnetales, Bennettitales, and angiosperms, can be added pollen sacs borne in aggregates of two or more (Figs. 3.45C,F,I and 3.44A,B,D,E,G,G',H), paired bracteoles that enclose the pollen sacs or are fused with them to form synangia (lost in Pentoxylales by reduction?; Figs. 3.45C,F,I and 3.44G,H), microsporophylls borne on perianth parts or on the macrocupule derived from perianth parts (lost in Pentoxylales by reduction?; Figs. 3.45A,D,G), and simple tectate-granular, monosulcate pollen (this last character was assumed for the Gnetales before the discovery of Archaestrobilus).
Figure 3.45 shows how the basic microsporophyll units of angiophytes (Fig. 3.45C), gnetophytes (Fig. 3.45F), and bennettitaleans (Fig. 3.451) could be derived from an ancestral sporangiophore subtended by a pair of bracts or bracteoles. In gnetophytes, the pollen sacs remained integral on a filamentous axis. Even when the number of pollen sacs and axial length is reduced, the bracteoles remain free rand unfused to that axis, or to the sacs. In Sahnia (microsporangiate flower of Pentoxylon), the axis either remained elongate and branched (vestigial plesiomorphy?) or became so. If bracteoles were present in early developmental stages, they are not present in mature specimens (Crane, 1985). In bennettitaleans the axis was lost and the pollen sacs became fused to the adaxial sides of the bracteoles. This condition was apparently modified in some early Bennettitales, in which the pollen sacs are not clearly borne in bivalved synangia; for example, Bennettistemon, Haitingeria, Leguminanthus, and Leuthardtia (Crane, 1986). Thus, in the Pentoxylales and perhaps also the Bennettitales, bracteoles were lost in some taxa early in anthophyte history, whereas the supporting axis was retained in most taxa where pollen sac fusion with the bracteoles did not occur.
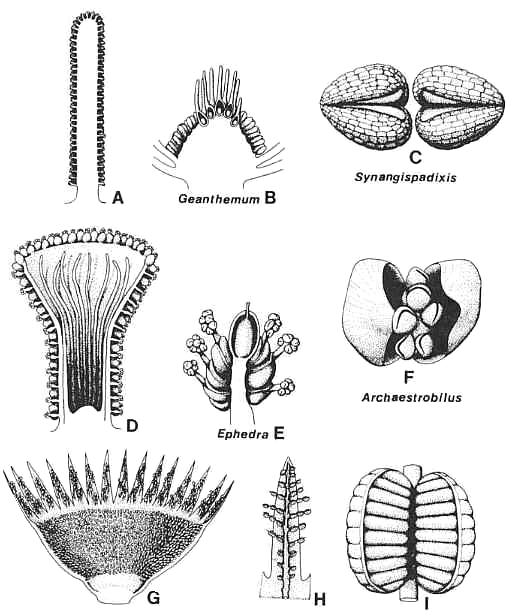
Figure 3.45. Comparison of anthophyte microsporangiate organs with those of Archaestrobilus. (A) Synangispadixis tidwellii male flower, longitudinal section. (B) Geanthemum bisexual flower, longitudinal section, showing. sessile stamens attached below collective bases (receptacle) of carpels/macrocupules. (C) S. tidwellii, tetraloculate anthers without connective, drawn to show-origin from a pair of conduplicate bracteoles. (D) Archaestrobilus cupulanthus, longitudinal section showing distribution of bivalved microsporophylls on outside of macrocupule and reduced sterile (interseminal) scales on inside. (E) Ephedra campylopoda, showing reduced bisexual inflorescence and male macrocupules extended beyond enclosing paired bracts. (F) A. cupulanthus bivalved microsporophyll, showing axile position of stalked pollen-sac apparatus encompassed by pair of bracteoles; an analogous morphology (higher order of complexity) is duplicated by Ephedra male flowers (E). (G) Weltrichia sol, longitudinal-half section through male flower, redrawn from Crane (1985, Fig. 10D). (H) Weltrichia spectabilis, detail of branched bivalved microsporophylls attached to a perianth lobe; redrawn after Stewart and Rothwell (1993, Fig. 3.25.7C). (I) Weltrichia sol, detail of bivalved synangium; redrawn after Crane (1985, Fig. 10E).
Information on the seeds and the pollen organ of the Eucommiidites-producing plant indicates a possible anthophyte affinity (Pedersen et al., 1989a). Those characters that support this relationship are ovules with two integuments (Erdtmanispermum) and spherical heads (Erdtmanitheca) containing hundreds of radiating pollen sacs borne in probable synangia. Because of the morphology of its seeds and its triaperturate (modified polyplicate?) pollen, this taxon has been compared to the Gnetales. It is, therefore, important to recognize diagnostic differences between Erdtmanitheca and Archaestrobilus. The apparent fusion of numerous elongate pollen sacs with a bract-like organ and their position inside that organ (Pedersen et al., 1989a) compares more with the condition found in the Bennettitales than it does with that of the Gnetales and Archaestrobilus, where the pollen sacs are free and unfused to associated bracteoles or bracts. There is greater similarity between Erdmanitheca and Synangispadixis male flowers in which the macrocupule has been reduced to a supporting axis (pseudoaxis).
In angiophytes (represented by the stem angiophyte Sanmiguelia lewisii/ Synangispadixis tidwellii) the pollen sacs became fused to the adaxial sides of the enclosing bracteoles, and the pollen sac cuticle was reduced, like that of most Bennettitales (Fig. 3.45C). In addition to the formation of synangia, the bractioles became plicate or conduplicate. In folding, they almost completely enclosed the pollen sacs. With conduplication and space limitation, the number of pollen sacs was reduced from many to two functional sacs per bracteole. The reduced median pollen sacs were not completely lost but were apparently modified to form a septum that separates the two outermost sacs. That interpretation would explain why the septum disappears with pollen maturity as do the pollen sac walls [no pollen sac cuticle can be demonstrated for Synangispadixis (Cornet, 1986, 1989b)]. Each conduplicate bracteole with paired pollen sacs is identical in construction to an angiosperm anther. I propose that the presence of an ephemeral septum separating two pollen sacs per biacteole plus the paired nature of the bracteoles (paired anthers give tetraloculate condition for angiosperms) represents the hallmark synapomorphy (symplesiomorphy) for all angiosperms (Cornet, 1989b).
If there is any single reason why Sanmiguelia cannot be placed in the gnetophytes or in any other recognized nonangiophyte sister group, this is the most important one. Not everyone may agree with this assessment: Doyle and Donoghue (1993), for example, did not recognize or accept the paired nature of the biloculate anthers and compared Synangispadixis with ginkgophyte strobili bearing sporophylls with two pollen sacs. Crane (personal communication, 1993), however, concurs with my interpretation.
Homologies Among Anthophyte Gynoecia
Recognition of rudimentary scales or reduced bracteoles at the base of the aborted ovule in the male flower of Welwitschia (Fig. 3.44D: Martins, 1971) has important implications: I interpret the scales surrounding the ovule in Archaestrobilus to be homologous with the rudimentary scales in Welwitschia (Fig. 3.44D). Their similarity in shape and position to interseminal (sterile) scales around ovules in bennettitalean flowers cannot be ignored (compare Fig. 3.46D,G,I). Some ovuliferous macrocupules of Archaestrobilus were found with nodular structures at the base of some of the sterile scales (Fig. 3.19, arrow; Fig. 3.46D). They may well represent aborted or rudimentary ovules and not just sterile scales with mineral deposits, and thus be derived from a multiovulate bennettitaiean-like flower (cf. Takhtajan, 1969; Ehrendorfer, 1976). However, the presence of such accessory ovules is not necessary for the elucidation of homologies, only helpful.
The nucellus in Gnetum is surrounded by three tubular envelopes (Martens, 1971; Crane, 1985). The middle envelope may be derived from the coalescence or fusion of sterile scales (analogous to the fusion of paired bracteoles around the anther stalk?). If this interpretation is correct, only Ephedra would lack a structure homologous with the middle envelope of the Gnetum ovule and/or the rudimentary scales of Welwitschia. The possible relationship of the middle envelope to plesiomorphic organs is the kind of understanding that can be derived only from the fossil record. Taken together with the possible plesiomorphic morphology of Gnetum microsporophylls (see above), Doyle and Donoghue's (1986b, Fig. 14d) entertainment of a cladistic tree where Gnetum is basal on the gnetophyte branch is reasonable. For those who would interpret the dicot-like leaf (Pannaulika triassica) and flowers from the Late Triassic (Cornet, 1993) as gnetalean, the above possibility becomes a viable possibility. In opposition, one must consider mosaic evolution (Gnetum does not have to be advanced in all characters) and the much longer tree length (101 steps vs. 31-47 steps from the work Doyle and Donoghue, 1986b) required to place Gnetum before the other Gnetales. In addition, Doyle and Donoghue (1992) and Laconte and Stevenson (1990) regard leaf vein reticulation as not homologous in angiosperms and Gnetum (see also Trivett and Pigg, Chapter 2).
Crane (1985) and Doyle and Donoghuer (1986b) interpret female bennettitalean and pentoxylalean flowers as consisting of aggregates of uniovulate cupules separated by sterile scales (Fig. 3.46H,I). If the organization of cupular ovules and sterile scales is basic to the anthophytes (see also Taylor and Kirchner, Chapter 6), then the macrocupule in Archaestrobilus (and by homology the outer envelope or wing surrounding the ovules in extant Gnetales) may be homologous with the perianth of the bennettitalean flower (Doyle and Donoghue, 1986b: character 35). Such an interpretation contradicts that of Crane (1985), who homologizes the macrocupule of Welwitschia (his inner bracteoles) with the "perianth" of Ephedra and Gnetum.
My interpretation has the flowers of extant Gnetales and those of Archaestrobilus constructed on an identical bauplan with all bracts and envelopes accounted for by plesiomorphic characters (organs sometimes missing in extant taxa). The bennettitalean flower (exemplified by Williamsonia harrisiana: Fig. 3.46G), in this interpretation, represents the plesiomorphic condition for the anthophytes (cf. Doyle and Donoghue, 1986a). The only significant difference between the flowers of anthophytes is the reduction in the number of sterile and fertile parts.
Origin and Homologies of the Angiosperm Carpel and Stamen
With the acceptance of gnetopsids as the closest living sister group to the angiosperms, a conflict arose between the traditional phyllosporous interpretation of the carpel and the stachyosporous explanation of the bracteole-bearing axes terminated by an ovule in gnetophytes (Stevenson, 1993; Doyle and Donoghue, 1992). Complicating this is the interpretation by Taylor (1991; see also Taylor and Kirchner, Chapter 6) that the most basic of angiosperm carpels is ascidiate with a single basal, orthotropous ovule. Cornet (1986) attempted to explain the morphology of the Axelrodia carpel (Late Triassic) by considering a similar origin via the enclosure of a terminal ovule-bearing branch by a subtending clasping bract or leaf. If the macrocupule of Archaestrobilus is a reduced uniovulate version of the bennettitalean flower, then the macrocupule is probably homologous with the inner whorl of bracts in, for example, Williamsonia (compare Figs. 3.46D and 3.46G). The presence of a ventral suture on the macrocupule of Archaestrobilus implies that it is derived from either a large encircling bract or from two or more bracts, all of whose margins became fused except along one side. Because the suture does not seem to have importance in dehiscence (the ovule was enclosed by sterile scales) and the suture is apical in orientation, the former interpretation is preferred because it is a by-product of an origin from a single bract. Continued interpretation of the outer envelope of the gnetalean ovule as being derived from a pair of bracts is not supported by development or by Archaestrobilus. The angiosperm carpel can be either conduplicate with an extended suture or ascidiate with no suture in primitive living angiosperms; similarly, the loss of the suture in extant Gnetales is interpreted as an apomorphy, with no more or less significance than its closure in angiosperms. The evolution of the angiosperm carpel from such a macrocupule is considered here to be the most parsimonious interpretation (see also Cornet, 1989b).
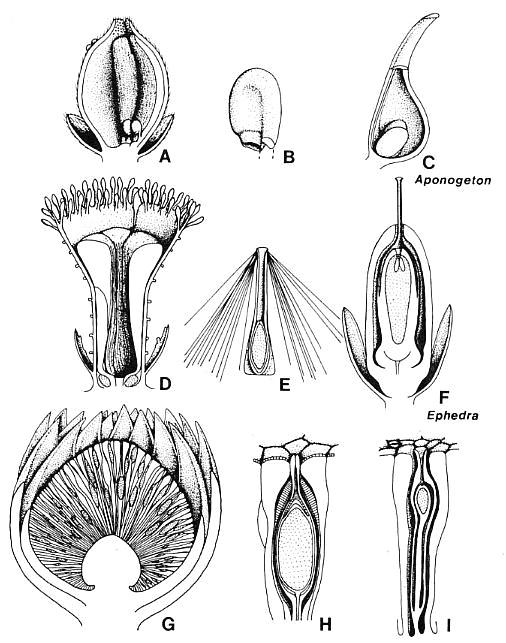
Figure 3.46. Comparison of anthophyte megasporangiate organs with those of Archaestrobilus. (A) Axelrodia burgeri carpel/macrocupule, longitudinal section showing two anatropous ovules attached to base of a pair of sterile scales, one on either side of ventral suture. Papillae of "transmission tissue" on ovary side of sterile scales; papillae and hairs around stigmatic apex (based on Cornet 1986, 1989b). Gland-like organs as round bumps near apex on left side, and portion of bract-like perianth also shown. (B) A. burgeri anatropous ovule showing two integuments (based on Cornet, 1989b). (C) Aponogeton (monocot) carpel with pair of basal ovules shown for comparison. (D) Archaestrobilus cupulanthus, longitudinal section showing macrocupule with gland-like bodies around rim, ventral suture, ring of sterile scales (some with possible aborted ovules) surrounding central cavity for a single seed, and some of the digitate bracts around the base of the macrocupule. (E) Reconstruction of A. cupulanthus seed, based on specimens from Dan River/Danville basin, North Carolina (Fig. 3.20). (F) Ephedra viridis female flower, longitudinal section with emphasis on homologies with Archaestrobilus flower in (D); redrawn from Chamberlain (1966, Fig. 356). (G) Williamsonia harrisiana female flower, showing gynoecium with numerous cupulate ovules and sterile scales surrounded by bracteate perianth; redrawn from Crane (1985, Fig. 10A). (H) Cycadeoidea morierei ovule, longitudinal section showing interseminal scales enclosing a cupulate ovule with apical micropyle; redrawn from Stewart and Rothwell (1993, Fig. 25.10B). (I) Cycadeoid ovule with interseminal scales, redrawn and modified from Stewart (1993, Fig. 25.3A).
If the angiosperm carpel and gnetalean macrocupule evolved from a single bract, its larger size and subtending position to one or more ovules helped it to exclude other lower or outside bracts in a phyllotactic spiral or whorl. Development of such an enlarged bract over others in the perianth may be the result of size reduction, where the gynoecium was reduced from many ovules to one or two. In the Bennettitales (Fig. 3.46G) bract size and shape in the perianth remained similar. The constriction of the macrocupule apex to protect two or more ovules inside created the carpel and led to the evolution of the stigma in angiophytes (Fig. 3.46A). In the Gnetales, a single ovule with an extended micropyle obviated the necessity for a stigma when the macrocupule apex became constricted as in Ephedra (Fig. 3.44C).
In both clades, the ancestral condition (also for all anthophytes) was a bisexual macrocupule: Microsporophylls were attached to the outside of the macrocupule, whereas megasporophylls were contained within it. Differentiation into unisexual flowers probably evolved as a means to increase outcrossing before carpel closure and the development of sporophytic incompatibility. Unlike the Gnetales, retention of two or more ovules (or the potential for more than one ovule) and a shift from prefertilization to postfertilization development of the ovule resulted in additionai specializations in angiosperms: (1) interseminal (sterile) scales modified to form a pollen tube transmission tissue that connects stigma with ovules (Fig. 3.46A; Cornet, 1989b, Fig. 9); (2) ovules that became anatropous so that the micropyle would face the transmission tissue (Fig. 3.46B); and (3) development of bilateral rather than radial symmetry for the arrangement of paired ovules and transmission tissue tracts within the ovary (see also Taylor and Kirchner, Chapter 6). In the case of Axelrodia, the transmission tissue (rows of micropapillae aligned with axis of the carpel) developed on the ovary side of paired sterile scales, which became attached to the carpel wall, one on either side of the ventral suture (Cornet, 1989b, Fig. 9). If these interpretations are correct, that is, sterile scales are derived from aborted ovules, and transmission tissue from sterile scales, then pollen tubes in angiosperms follow ovule-derived structures from stigma to micropyle (Cornet, 1989b).
Evidence in support of some of these interpretations comes from the Chloranthaceae, considered to be among the most primitive living angiosperms (LeRoy, 1983b; Endress, 1987a; Todzia, 1988). Cornet (1989b) interpreted the angiosperm stamen to be homologous with the entire male flower of Synangispadixis, but that seems to involve too much reduction and modification. A more parsimonious interpretation is that the only thing lacking in the tetraloculate anthers of Synangispadixis is the connective [the anthers of Synangispadixis are joined at their base by a cellular connection that may be the precursor to the connective and filament (Cornet, 1986, 1989b )]. Transposition of stamens from male to female flowers resulted in the secondary evolution of a bisexual flower (perhaps a contributing factor in mid-Cretaceous diversification?). Thus, the position of anthers/stamens borne on the outside of the carpel in Sarcandra is viewed as not exceptional: That is where they belong on a bisexual macrocupule. Subsequent refinement moved the stamens to the base of the carpel/ macrocupule (cf. Gnetum and Fig. 3.4Sb). As further evidence that the male flower of Synangispadixis is relevant, consider the male flower of Hedyosmum: It consists of an axis bearing hundreds of sessile stamens. It does not have to be interpreted as the product of extreme floral reduction (see LeRoy, 1983a, 1983b). It could represent the male counterpart of the bracteate carpel (as does the Synangispadixis male flower), for which the axis (i.e., pseudoaxis) is the carpel homolog. In this interpretation, the only significant distinctions between the male flowers of Hedyosmum and Sanmiguelia (Synangispadixis) are the evolution of a connective between the anthers, and the evolution of tectate-columellate pollen with multiple apertures. Thus, stamens are not homologs of carpels, but homologues of ovules plus transmission tissue (if derived from interseminal scales). Consistent with this interpretation, the connective or laminar part of the stamen evolved de novo, as did staminodial petals (Walker and Walker, 1984; cf. Friis et al., 1991).
CONCLUSIONS
Archaestrobilus cupulanthus gen. et sp. nov. from the late Carnian Trujillo Formation of Texas possesses combinations of characters shared with extant Gnetales, such as large strobili borne in clusters, groups of three bracts attached on the reproductive axis in a tight spiral and separated by long internodes (almost whorled), and complex, unisexual, cupulate flowers. The presence on the outside of the female macrocupule of gland-like structures that resemble the stalks that bear pollen sacs on the male macrocupule, and the presence of sterile filamentous appendages inside the male macrocupule that resemble the sterile scales around the ovule, imply an origin from a bisexual macrocupule. The presence of simple, ovoid monosulcate pollen, radially symmetric rather than bilaterally flattened macrocupules, fully developed sterile scales around the ovule, and paired bracteoles that enclose the pollen organs indicate a plant more primitive than extant Gnetales, but one that belongs in the gnetophytes and is probably a stem gnetophyte.
The association of Archaestrobilus with Pelourdea poleoensis leaves and stems suggests that they belong to the same plant. This latter taxon was herbaceous (also indicated by the occurrence of reproductive axes in a semiaquatic habitat), and was unlike angiophytes (see Ash, 1987) in being non-rhizomatous with long, spirally-arranged, strap-shaped leaves that possessed only two sizes of parallel-veins compared with four sizes in Sanmiguelia (Cornet, 1986). Large strobili probably borne terminally on primary rather than secondary axes, and orthotropous seeds with long tapering micropyles add to the characters that nest Archaestrobilus in the gnetophytes. Probable insect involvement in pollination and the presence of an apical tuft of hair-like appendages on the seed that served in wind dispersal are also consistent with a stem gnetophyte/anthophyte affinity.
Similarities in the construction of unisexual flowers between Archaestrobilus and Sanmiguelia (see also Cornet, 1989b) indicate a common ancestry for both taxa and stress the stachyosporous rather than phyllosporous origin of the angiosperm carpel and stamen. Comparison with Sarcandra and Hedyosmum (Chloranthaceae) reveals a similar floral organization and confirms the primitive status of these taxa among living angiosperms.
ACKNOWLEDGMENTS
The author thanks David Winship Taylor and Leo J. Hickey, the organizers of the symposium, for inviting me to participate. He also thanks Elizabeth B. Cornet (my mother) for help in collecting the specimens in 1986, Bonnie Lee Cornet (deceased 1991) for her unforgettable support during the early stages of this study, and Patricia Huff-Cornet for her unconditional support in making the completion of this chapter possible. I acknowledge the support of Paul E. Olsen, who encouraged me to make this contribution and supported me through his grants at Lamont-Doherty Earth Observatory during various stages of this study.
REFERENCES
Combined with references from other chapters at the end of the book (45 pages).
This web page was created on 10 June 2002; last updated on 05/15/2005.